
ЎҫМвДҝЎҝДіРЈ»ҜѧСЧйС§ЙъАыУГИзНјЛщБРЧ°ЦГҪшРРЎ°МъУлЛ®ХфЖш·ҙУҰЎұөДКөСйЈ¬ІўАыУГІъОпҪшТ»ІҪЦЖИЎFeCl3ЎӨ6H2Oҫ§МеЎЈ(НјЦРјРіЦј°ОІЖшҙҰАнЧ°ЦГҫщТСВФИҘ)

(1)Ч°ЦГBЦР·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪКЗ__________________________ЎЈ
(2)Ч°ЦГEЦРөДПЦПуКЗ_____________________________________ЎЈ
(3) ёГРЎЧйС§ЙъАыУГBЧ°ЦГөД№ММеУлЧгБҝСОЛб·ҙУҰәуөДВЛТәЦЖИЎFeCl3ЎӨ6H2Oҫ§МеЈ¬ЙијЖБчіМИзНјЛщКҫЈә
![]()
ўЩІҪЦиIЦРНЁИлCl2өДЧчУГКЗ______________________________ЎЈ
ўЪ јтКцјмСйВЛТәЦРFe3+өДІЩЧч·Ҫ·Ё________________________ЎЈ
ўЫІҪЦиўтҙУFeCl3ПЎИЬТәЦРөГөҪFeCl3ЎӨ6H2Oҫ§МеөДЦчТӘІЩЧч°ьАЁЈә___________ЎЈ
Ўҫҙр°ёЎҝ3Fe+4H2O(g)![]() Fe3O4+4H2 әЪЙ«өД·ЫД©ұдіЙЧПәмЙ«Ј¬№ЬұЪІъЙъЛ®Цй Ҫ«Fe2+Сх»ҜіЙFe3+ ИЎЙЩБҝВЛТәЈ¬өОИлјёөОKSCNИЬТәЈ¬№ЫІмИЬТәКЗ·сұдәмЙ« јУИИЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛ
Fe3O4+4H2 әЪЙ«өД·ЫД©ұдіЙЧПәмЙ«Ј¬№ЬұЪІъЙъЛ®Цй Ҫ«Fe2+Сх»ҜіЙFe3+ ИЎЙЩБҝВЛТәЈ¬өОИлјёөОKSCNИЬТәЈ¬№ЫІмИЬТәКЗ·сұдәмЙ« јУИИЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛ
ЎҫҪвОцЎҝ
ЈЁ1Ј©МъУлЛ®ХфЖшёЯОВПВ·ҙУҰЙъіЙЛДСх»ҜИэМъәНЗвЖшЈ¬ҫЭҙЛРҙіц·ҙУҰөД»ҜС§·ҪіМКҪЈ»
ЈЁ2Ј©Ч°ЦГEЦРәЪЙ«өДСх»ҜНӯұ»ЗвЖш»№ФӯіЙәмЙ«өДНӯЈ¬Н¬КұУРЛ®ЦйЙъіЙЈ»
ЈЁ3Ј©ўЩёщҫЭБчіМҪбәПВИЖшөДЗҝСх»ҜРФ·ЦОцВИЖшөДЧчУГЈ»
ўЪёщҫЭИэјЫМъөДјмСй·Ҫ·ЁјУKSCNИЬТәЈ¬№ЫІмКЗ·сұдәмЈ»
ўЫУЙFeCl3ПЎИЬТәЦРөГөҪFeCl36H2Oҫ§МеРијУИИЕЁЛхЎўАдИҙҫ§МеЈ¬№эВЛЎЈ
ЈЁ1Ј©Ч°ЦГBЦРМъ·ЫУлЛ®ХфЖшФЪёЯОВПВ·ўЙъ·ҙУҰЙъіЙЛДСх»ҜИэМъәНЗвЖшЈ¬·ҙУҰөД»ҜС§·ҪіМКҪОӘЈә3Fe+4H2O(g)![]() Fe3O4+4H2Ј»
Fe3O4+4H2Ј»
ЈЁ2Ј©Ч°ЦГBМъУлЛ®ХфЖш·ҙУҰЙъіЙөДЗвЖшЈ¬ҫӯјоКҜ»ТёЙФпәујУИлЧ°ЦГEЈ¬Сх»ҜНӯУлЗвЖшјУИИ·ўЙъ·ҙУҰЙъіЙБЛНӯәНЛ®Ј¬ЛщТФ·ҙУҰөДПЦПуОӘЈәәЪЙ«өД·ЫД©ұдіЙЧПәмЙ«Ј¬№ЬұЪІъЙъЛ®ЦйЈ»
ЈЁ3Ј©BЧ°ЦГЦР·ҙУҰ»мәПОпЦРУРFe3O4Ј¬ҝЙДЬУРFeКЈУаЈ¬УлЧгБҝөДСОЛб·ҙУҰәу№эВЛЈ¬ВЛТәЦРә¬УРFeCl2әНFeCl3ЎЈўЩТтОӘВИЖшҫЯУРЗҝСх»ҜРФЈ¬ЛщТФДЬҪ«¶юјЫМъАлЧУСх»ҜОӘИэјЫМъАлЧУЈ¬№Кҙр°ёОӘЈәҪ«Fe2+Сх»ҜіЙFe3+Ј»
ўЪјмСйИэјЫМъУГKSCNИЬТәЈ¬№ЫІмКЗ·сұдәмЈ¬№Кҙр°ёОӘЈәИЎЙЩБҝВЛТәЈ¬өОИлјёөОKSCNИЬТәЈ¬№ЫІмИЬТәКЗ·сұдәмЙ«Ј»
ўЫУЙFeCl3ПЎИЬТәЦРөГөҪFeCl36H2Oҫ§МеРијУИИЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛЈ¬№Кҙр°ёОӘЈәјУИИЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝёщҫЭИзНј»ШҙрЈ¬ПВБРЛө·ЁІ»ХэИ·өДКЗ

A. ҙЛЧ°ЦГУГУЪМъұнГж¶ЖНӯКұЈ¬aОӘМъ
B. ҙЛЧ°ЦГУГУЪөз¶ЖНӯКұЈ¬БтЛбНӯИЬТәөДЕЁ¶ИІ»ұд
C. ИјБПөзіШЦРХэј«·ҙУҰОӘO2Ј«4eЈӯЈ«4HЈ«![]() 2H2O
2H2O
D. ИфУГёГЧ°ЦГҪшРРҙЦНӯөДҫ«Б¶Ј¬өұУР1 molЛ®ЙъіЙКұЈ¬ҝЙөГөҪ64 gҫ«Нӯ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіОВ¶ИПВЈ¬ФЪ 2LөДГЬұХИЭЖчЦРЈ¬јУИл1molX(g)әН 2molY(g)·ўЙъ·ҙУҰЈәX(g)+mY(g)3Z(g) ҰӨH=-Q kJЎӨmolЎҘ1(QЈҫ0)Ј¬10minәуёГ·ҙУҰҙпөҪЖҪәвКұЈ¬XЎўYөДОпЦКөДБҝ·ЦұрОӘ0.9molЎў1.8molЎЈПВБРРрКцІ»ХэИ·өДКЗ
A.m=2
B.ФЪ0~10minДЪЈ¬XөД·ҙУҰЛЩВКОӘ 0.005molЎӨLЎҘ1ЎӨminЎҘ1
C.10minәуЈ¬XөДПыәДЛЩВКөИУЪYөДЙъіЙЛЩВК
D.ФЪ0~10minДЪЈ¬XәН Y·ҙУҰ·ЕіцөДИИБҝОӘ 0.1Q kJ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝМјј°Жд»ҜәПОпУР№г·әөДУГНҫ
(1)јЧҙјіЈЧчИјБПЈ¬ТСЦӘФЪіЈОВіЈС№ПВјЧҙјНкИ«ИјЙХөДИИ»ҜС§·ҪіМКҪИзПВЈә2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(l) ҰӨH1ЎЈФт2molјЧҙјНкИ«ИјЙХЙъіЙ¶юСх»ҜМјәНЖшМ¬Л®Кұ·ҙУҰөДҰӨH______ҰӨH1(МоЎ°ҙуУЪЎұЎўЎ°өИУЪЎұ»тЎ°РЎУЪЎұ)
(2)ГәөДЧЫәПАыУГГәЖш»ҜКЗҪ«Л®ХфЖшНЁ№эәмИИөДМјІъЙъЛ®ГәЖшЈәC(s)+H2O(g)CO(g)+H2(g) ҰӨH=+131.3kJЎӨmolЎҘ1Ј¬ҙпөҪЖҪәвәуЈ¬ПВБРДЬМбёЯЛ®ГәЖшЙъіЙЛЩВКөДҙлК©КЗ______ЎЈ
A.ЙэёЯОВ¶И B.ФцјУМјөДУГБҝ C.ЛхРЎМе»э D.УГCOОьКХјБіэИҘCO
(3)Т»¶ЁОВ¶ИПВЈ¬Ҫ«Т»¶ЁБҝөДC(s)әНH2O(g)·ЕИлДіәгИЭГЬұХИЭЖчЦР·ўЙъЙПКц·ҙУҰЈ¬өГөҪИзПВКэҫЭЈә
ҙпөҪЖҪәвЛщРиКұјд/s | ЖрКјЕЁ¶И/ molЎӨLЎҘ1 | ЖҪәвЕЁ¶И/ molЎӨLЎҘ1 |
H2O(g) | H2(g) | |
10 | 2.0 | 0.6 |
јЖЛгёГ·ҙУҰҙУҝӘКјөҪЖҪәвКұЈ¬ТФCOұнКҫөД·ҙУҰЛЩВКОӘ______Ј»H2O(g)өДЧӘ»ҜВКОӘ______ЎЈ
(4)ПВБРҝЙТФЛөГч·ҙУҰC(s)+H2O(g)CO(g)+H2(g)ТСҫӯҙпөҪЖҪәвЧҙМ¬өДКЗ____ЎЈ
A.өҘО»КұјдДЪЙъіЙnmolH2өДН¬КұЈ¬ЙъіЙnmolCO
B.·ҙУҰЛЩВКЈә (H2)= (CO)= (H2O)
C.c(H2O):c(CO):c(H2)=1:1:1
D.ОВ¶ИәНМе»эТ»¶ЁКұЈ¬»мәПЖшМеөДГЬ¶ИІ»ФЩұд»Ҝ
E.ОВ¶ИәНМе»эТ»¶ЁКұЈ¬ИЭЖчДЪөДС№ЗҝІ»ФЩұд»Ҝ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ(1)јшұрKClИЬТәәНK2CO3өДКФјБГыіЖКЗ_______Ј¬АлЧУ·ҪіМКҪОӘ_____________ЎЈ
(2)іэИҘ»мИлNaClИЬТәЦРЙЩБҝNaHCO3ФУЦКөДКФјБГыіЖКЗ_______________Ј¬АлЧУ·ҪіМКҪОӘ_____________________________ЎЈ
(3)іэИҘNa2CO3·ЫД©ЦР»мИлөДNaHCO3ФУЦКУГ___________·Ҫ·ЁЈ¬»ҜС§·ҪіМКҪОӘ______________________________________________ЎЈ
(4)іэИҘМъ·ЫЦР»мУРВБ·ЫөДКФјБГыіЖКЗ_______Ј¬»ҜС§·ҪіМКҪОӘ_______________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіРЛИӨРЎЧйөДН¬С§УГНјЛщКҫЧ°ЦГСРҫҝУР№Шөз»ҜС§өДОКМвЈ®өұұХәПёГЧ°ЦГөДөзјьКұЈ¬№ЫІмөҪөзБчјЖөДЦёХл·ўЙъБЛЖ«ЧӘЈ®

Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©јЧіШОӘ______МоЎ°ФӯөзіШЎұЎўЎ°өзҪвіШЎұ»тЎ°өз¶ЖіШЎұ)Ј¬НЁИл CH3OH өзј«өДөзј«·ҙУҰОӘ_____Ј®
ЈЁ2Ј©ТТіШЦР A(КҜД«)өзј«өДГыіЖОӘ_____(МоЎ°Хэј«ЎұЎўЎ°ёәј«Ўұ»тЎ°Тхј«ЎұЎўЎ°Сфј«Ўұ)Ј¬ЧЬ·ҙУҰКҪОӘ_____Ј®
ЈЁ3Ј©өұТТіШЦР B ј«ЦКБҝФцјУ 5.40g КұЈ¬јЧіШЦРАнВЫЙППыәД O2 өДМе»эОӘ_____mL(ұкЧјЧҙҝц)Ј¬ұыіШЦР_____ј«Оціц_____g НӯЈ®
ЈЁ4Ј©ИфұыЦРөзј«І»ұдЈ¬Ҫ«ЖдИЬТә»»іЙ NaCl ИЬТәЈ¬өзјьұХәПТ»¶ОКұјдәуЈ¬ұыЦРИЬТәөД pH Ҫ«______________МоЎ°ФцҙуЎұЎўЎ°јхРЎЎұ»тЎ°І»ұдЎұ)Ј»јЧЦРИЬТәөД pH Ҫ«________________ (МоЎ°ФцҙуЎұЎўЎ°јхРЎЎұ»тЎ°І»ұдЎұ)Ј®
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТСЦӘЈәAЎўBЎўFКЗјТНҘЦРіЈјыөДУР»ъОпЈ¬FіЈУГУЪКіЖ·°ьЧ°Ј¬EКЗКҜУН»Ҝ№Ө·ўХ№Л®ЖҪөДұкЦҫЎЈёщҫЭПВГжЧӘ»Ҝ№ШПө»ШҙрОКМвЎЈ

ЈЁ1Ј©·ЦұрРҙіцAәНEЦР№ЩДЬНЕөДГыіЖЈәAЦР_________Ј»EЦР_________Ј»
ЈЁ2Ј©ІЩЧчўЮөДГыіЖОӘ________________ЎЈ
ЈЁ3Ј©Рҙіц·ҙУҰАаРНЈәўЬ_________Ј»
ЈЁ4Ј©ЗлРҙіцПВБР·ҙУҰөД»ҜС§·ҪіМКҪЈә
ўЩРҙіцAәНBФЪЕЁБтЛбЦРјУИИ·ҙУҰөД·ҪіМКҪ_________________Ј»
ўЪBФЪҪрКфНӯҙжФЪПВФЪҝХЖшЦРјУИИ·ҙУҰ________________Ј»
ЈЁ5Ј©FКЗТ»ЦЦіЈјыөДёЯ·ЦЧУІДБПЈ¬ЛьёшОТГЗҙшАҙБЛҫЮҙуөД·ҪұгЎЈИ»¶шЈ¬ХвЦЦІДБПФміЙөДөұҪсөДДіТ»»·ҫіОКМвКЗ__________________.
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіОВ¶ИПВЈ¬Пт10 mL ![]() ИЬТәЦРөОјУ
ИЬТәЦРөОјУ![]() өД
өД![]() ИЬТәЈ¬өОјУ№эіМЦРЈ¬ИЬТәЦР
ИЬТәЈ¬өОјУ№эіМЦРЈ¬ИЬТәЦР![]() Ул
Ул![]() ИЬТәМе»э(V)өД№ШПөИзНјЛщКҫЈ¬ПВБРЛө·ЁХэИ·өДКЗ( )ТСЦӘЈә
ИЬТәМе»э(V)өД№ШПөИзНјЛщКҫЈ¬ПВБРЛө·ЁХэИ·өДКЗ( )ТСЦӘЈә ![]() ЎЈ
ЎЈ
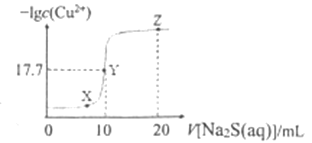
A. ёГОВ¶ИПВ ![]()
B. XЎўYЎўZИэөгЦРЈ¬YөгЛ®өДөзАліМ¶ИЧоРЎ
C. ![]() ИЬТәЦРЈә
ИЬТәЦРЈә![]()
D. Пт100 mL ![]() ЕЁ¶ИҫщОӘ
ЕЁ¶ИҫщОӘ![]() өД»мәПИЬТәЦРЦрөОјУИл
өД»мәПИЬТәЦРЦрөОјУИл![]() өД
өД![]() ИЬТәЈ¬
ИЬТәЈ¬![]() ПИіБөн
ПИіБөн
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝолНӯКЗ№г·әУҰУГУЪЦЖФмёЯј¶өҜРФФӘјюөДБјәГәПҪрЎЈДіҝЖСРРЎЧйҙУДі¶ИҫЙолНӯФӘјю(ә¬25%BeOЎў71%CuSЎўЙЩБҝFeSәНSiO2)ЦР»ШКХоләННӯБҪЦЦҪрКфөД№ӨТХБчіМИзПВЈә

ТСЦӘЈәI.олЎўВБФӘЛШ»ҜС§РФЦКПаЛЖЈ»
ўт.іЈОВПВІҝ·ЦДСИЬОпөДИЬ¶И»эіЈКэИзПВұнЈә

(1)ВЛТәAөДЦчТӘіЙ·ЦіэNaOHНвЈ¬»№УР_____________(Мо»ҜС§КҪ)Ј¬Рҙіц·ҙУҰIЦРә¬ол»ҜәПОпУл№эБҝСОЛб·ҙУҰөДАлЧУ·ҪіМКҪЈә_______________________________________________ЎЈ
(2)ўЩВЛТәCЦРә¬NaClЎўBeCl2әНЙЩБҝHClЈ¬ОӘМбҙҝBeCl2Ј¬ЧоәПАнөДКөСйІҪЦиЛіРтОӘ_______(МоЧЦДё)
a.јУИл№эБҝөД°ұЛ® b.НЁИл№эБҝөДCO2
c.јУИл№эБҝөДNaOH d.јУИлККБҝөДHCl e.ПҙөУ f.№эВЛ
(3)MnO2ДЬҪ«ҪрКфБт»ҜОпЦРөДБтФӘЛШСх»ҜОӘБтөҘЦКЈ®Рҙіц·ҙУҰўтЦРCuS·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪЈә____________________________________________ЎЈ
(4)ВЛТәDЦРc(Cu2+)=2.2molЎӨLЈӯ1Ўўc(Fe3+)=0.008molЎӨLЈӯ1Ўўc(Mn2+)=0.01molЎӨLЈӯ1Ј¬ЦрөОјУИлПЎ°ұЛ®өчҪЪpHҝЙҪ«ЖдТАҙО·ЦАлЈ¬КЧПИіБөнөДКЗ___________(МоАлЧУ·ыәЕ)Ј¬ОӘК№НӯАлЧУҝӘКјіБөнЈ¬іЈОВПВУҰөчҪЪИЬТәөДpHҙуУЪ___________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com