�������ʼ��仯�����빤ũҵ�������ճ����������е���ϵ����ش��������⣺
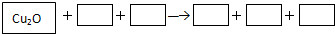
��1����֪�����Ȼ�ѧ����ʽ��
Fe
2O
3��s��+3CO��g���T2Fe��s��+3CO
2��g����H
1=-25kJ?mol
-13Fe
2O
3��s��+CO��g���T2Fe
3O
4��s��+CO
2��g����H
2=-47kJ?mol
-1Fe
3O
4��s��+CO��g���T3FeO��s��+CO
2��g����H
3=+19kJ?mol
-1д��FeO��s����CO��g����ԭ����Fe��s����CO
2��g�����Ȼ�ѧ����ʽ
��
��2���ӿ���ѧ���ϲ��һ����������Ȼ��������·�Ӧ��14CuSO
4+5FeS
2+12H
2O�T7Cu
2S+5FeSO
4+12H
2SO
4����5mol FeS
2������Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ
��
��3��һ�������£���Fe
2O
3��NiO��Cr
2O
3��������ȼú����������ӦΪ��
2CO��g��+SO
2��g��
2CO
2��g��+S��l����H=-270kJ?mol
-1����������ͬ��������ͬʱ��SO
2��ת�����淴Ӧ�¶ȵı仯��ͼ1��Fe
2O
3��NiO����������ʹSO
2��ת���ʴﵽ��ߣ������Ǵ����۸����أ�ѡ��Fe
2O
3����Ҫ�ŵ��ǣ�
��
ij��������Fe
2O
3����������380��ʱ���ֱ��о���[n��CO����n��SO
2��]�ֱ�Ϊ1��1��3��1ʱSO
2ת���ʵı仯�����ͼ2������ͼ2�б�ʾn��CO����n��SO
2��=3��1�ı仯����Ϊ
��
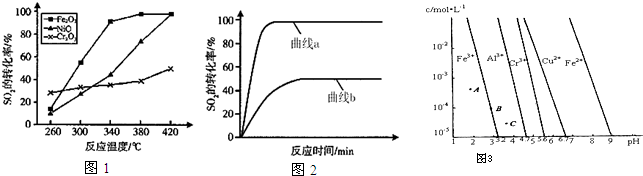
��4��ͨ��������Һ��pH�Թ�ҵ��ˮ�еĽ������ӽ��з��룮ͼ3��ijЩ�������������ڲ�ͬŨ�Ⱥ�pHʱ�ij���--�ܽ�ͼ��ͼ��ֱ���ϵĵ��ʾƽ��״̬������Һ�е�����Ũ��С��1��10
-5mol?L
-1ʱ����Ϊ�����ӳ�����ȫ��
����ͬ�����£�Fe��OH��
3��Al��OH��
3��Cr��OH��
3�������ʵ��ܶȻ�����������
��ͼ3��A��B��C�����б�ʾFe��OH��
3�ij������ʴ����ܽ����ʵ���
��
����ͼ3�ɵ�Fe��OH��
2���ܶȻ���ֵΪ
��
��5��LiFePO
4��ؾ����ȶ��Ըߡ���ȫ���������ŵ㣬�����ڵ綯��������ط�ӦΪ��FePO
4+Li
LiFePO
4����ص�����������LiFePO
4������������ʯī����Li
+�������Ϊ����ʣ��ŵ�ʱ���������ӦΪ
��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�