
ij�¶�����ˮ�д������µ� ��ƽ�⣺D2O
��ƽ�⣺D2O  D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ� ��
D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ� ��
A 0.1 molNaOD ������ˮ�γ�1L��Һ����pD=11
B��pD=4��DCl����ˮ��Һ������ˮϡ��100���� ��pD=6
C ��30 ml 0.5mol/L NaOD ����ˮ��Һ�м���20ml 0.5mol/LDCl����ˮ��Һ��������Һ��pD=11
D pD=10��NaOD��ˮ��Һ�У�����ˮ�������C��OD-��=1��10-10mol/L

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ��ɫ��У�����ڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
�״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϡ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��

��1����Ӧ����?????? ��������������������������Ӧ��
��2��ij�¶��·�Ӧ����H2��ƽ��ת���ʣ�a������ϵ��ѹǿ(P)�Ĺ�ϵ����ͼ��ʾ����ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K(A)????????? K(B)�������������������������������ݷ�Ӧ���������Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3=?????? ����K1��K2��ʾ����

��3����3 L�ݻ��ɱ���ܱ������з�����Ӧ������֪c(CO)�뷴Ӧʱ��t�仯��������ͼ��ʾ������t0ʱ�̷ֱ�ı�һ����������������Ϊ����������������

����������Ϊ������ʱ���ı��������??????????????? ��
����������Ϊ������ʱ���ı��������??????????????? ��
��4���״�ȼ�ϵ�����Ź㷺����;��ͬʱAl-AgO�����Ӧ�ù㷺������? �أ���ԭ����ͼ��ʾ���õ�صĸ�����Ӧʽ��???????? ��

��5��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣͨ��״���£���a mol/L�Ĵ�����b mol/LBa(OH)2��Һ�������ϣ���Ӧƽ��ʱ��2c(Ba2��)= c(CH3COO��)���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ??????????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ��������У�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ѡ����
ij�¶�����ˮ�д������µ���ƽ�⣺D2O  D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ�
��
D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ�
��
A 0.1 molNaOD ������ˮ�γ�1L��Һ����pD=11
B��pD=4��DCl����ˮ��Һ������ˮϡ��100���� ��pD=6
C ��30 ml 0.5mol/L NaOD ����ˮ��Һ�м���20ml 0.5mol/LDCl����ˮ��Һ��������Һ��pD=11
D pD=10��NaOD��ˮ��Һ�У�����ˮ�������C��OD-��=1��10-10mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
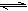 D++OD-��KW��D2O��=1.0��10-12mol2?L-2����pD=-lg��D+�ݣ����ڸ��¶��£�
D++OD-��KW��D2O��=1.0��10-12mol2?L-2����pD=-lg��D+�ݣ����ڸ��¶��£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶�����ˮ�д������µ���ƽ�⣺D2O D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ� ��
A 0.1 molNaOD ������ˮ�γ�1L��Һ����pD=11
B��pD=4��DCl����ˮ��Һ������ˮϡ��100���� ��pD=6
C ��30 ml 0.5mol/L NaOD ����ˮ��Һ�м���20ml 0.5mol/LDCl����ˮ��Һ��������Һ��pD=11
D pD=10��NaOD��ˮ��Һ�У�����ˮ�������C��OD-��=1��10-10mol/L
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com