
��ҵ���ô�����ͭ(������FeO)Ϊԭ����ȡ�Ȼ�ͭ����(CuCl2��2H2O)�������������£�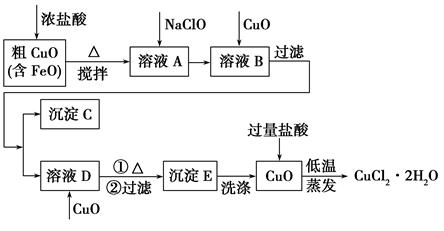
| ���� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH | ��9.6 | ��6.4 | 3��4 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡ����Fe2O3��ĩ(����ɫ)�����������ᣬ������Ӧ�Ļ�ѧ����ʽ�� ����Ӧ��õ�����Һ�� ɫ���ô���Һ�ֱ�������ʵ�飺
(1)ȡ������Һ�����Թ��У����뼸��NaOH��Һ���ɹ۲쵽�к��ɫ�������ɣ���Ӧ�Ļ�ѧ����ʽΪ ���˷�Ӧ���� (�Ӧ����)��
(2)��С�ձ��м���20 mL����ˮ�����������ں����ˮ�е��뼸�α���FeCl3��Һ�������������Һ�� ɫ�����Ƶ�Fe(OH)3���塣
(3)ȡ��һֻС�ձ�Ҳ����20 mL����ˮ�����ձ��м���1 mL FeCl3��Һ�����Ⱥ����ձ�(��ż�)��ʢ��Fe(OH)3������ձ�(�����)һ������ڰ������ֱ��ü���������ձ��е�Һ�壬���Կ��� �ձ��е�Һ����������ЧӦ�����ʵ������������� ��
(4)��Fe(OH)3�����������ʵ�飺
�ٽ���װ��U�ι��У���ʯī�缫��ֱͨ���磬ͨ��һ��ʱ�����������������ɫ�����˵�� �����������Ϊ ��
�������м��뱥��(NH4)2SO4��Һ������������ ��ԭ���� ��
�������е������ϡ���ᣬ������ ����ԭ���� ��
���ᴿ�˷�ɢϵ���õķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������CO��ԭ14 g����������Ļ����,�����ɵ�����ͨ�������ij���ʯ��ˮ��,���ɳ���25 g,�����ֻ��������Ǣ�FeO��Fe2O3����FeO��Fe3O4����Fe2O3��Fe3O4�е���������,�����ʵ���֮��Ϊ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������[KAl(SO4)2��12H2O]�Ʊ�Al��K2SO4��H2SO4���������£�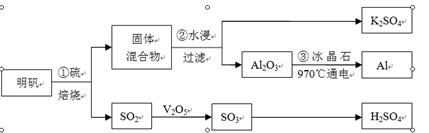
�������յĻ�ѧ����ʽΪ��4KAl(SO4)2��12H2O+3S��2K2SO4 +2Al2O3+9SO2��+48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У��������� ��
��2��������У�Ϊ��߽����ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
| A������������� | B�������¶� | C�����Ͻ��� | D�����̽���ʱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���(�����Ƽ)�Ͱ�����������̼�Ҫ��ʾ����ͼ��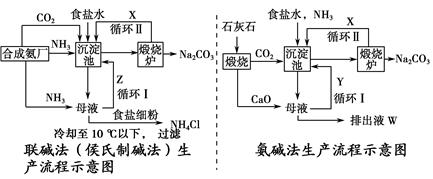
(1)�������з�����Ӧ�Ļ�ѧ����ʽΪ________________��
(2)X��________��Y��________(�ѧʽ)��
(3)Z�г����ܽ�İ�����ʳ���⣬�������ʻ���________________���ų�ҺW�е����ʳ������������⣬����________________��
(4)�������Ϸ������ڰ������������________(���Ҫ������Ҫ��)���䰱����
(5)��������д�ĸҺ����ȡ�Ȼ�茶���Ĺ����Ʋ⣬���ý�����ȷ��________(�����)��
a������ʱ�Ȼ�淋��ܽ�ȱ��Ȼ���С
b��ͨ�백��Ŀ����ʹ�Ȼ�笠�������
c������ʳ��ϸ��Ŀ�������Na����Ũ�ȣ��ٽ�̼�����ƽᾧ����
(6)����백���ȣ�ָ�������һ���ŵ�___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ճ�������ʹ�õ����Ͻ��е��������ڵ������������ҵ�ϵ��������Ҫ���䴿�Ȳ��õ���98.2%������Ȼ�����������������Ϊ50%��70%��������ҪΪSiO2��Fe2O3��CaO��MgO��Na2O�ȡ���ҵ���������Ĺ�������ʾ��ͼ���£�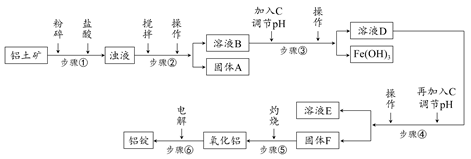
�� һЩ�������������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Mg(OH)2 |
| ��ʼ����pH(���ӳ�ʼŨ��0.01 mol/L) | 4 | 2.3 | 10.4 |
| ��ȫ����pH(����Ũ��<10��5 mol/L) | 5.2 | 4.1 | 12.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵؿ��к����ܷḻ��Ԫ�أ����������ڸ�¯��ұ���ģ���ұ��ҵ�У����ý�̿��ұ������
(1)д����̿�ڸ�¯���������������Ӧ�Ļ�ѧ����ʽ��
___________________________��
(2)д��������CO��ԭ�����Ļ�ѧ����ʽ��________________��
(3)ʵ�����п���CO��ԭFe2O3���ڼ���Fe2O3ǰӦ________��ֱ����________����ʱ���ܵ�ȼ�ƾ��Ƽ��ȣ�������ܷ���________����δ��Ӧ��ȫ��CO________(��ܡ����ܡ�)�ŷŵ������У�Ӧ��________������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������������������������������������ȣ�Ϊԭ���Ʊ�����{�ɱ�ʾΪFe2(OH)n(SO4)3-n/2�����̷�(FeSO4.7H2O)���гɱ��͡���Ӧ�졢��Ʒ�����ߵ��ŵ㡣�Ʊ���������ͼ��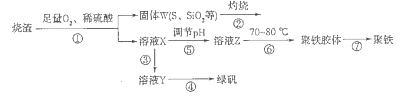
��1������ͼ����ҺXΪFe(SO4)3����Һ���ݴ�д��������������������ԭ��Ӧ�����ӷ���ʽ��____________��
��2�������ڵĴ������ڱˣ��ñ���_____________��������W��KOH��Һ���ϼ��ȣ�����һ����Ӧ�Ļ�ѧ����ʽΪ3S+6KOH 2K2S+K2SO3+3H2O���÷�Ӧ���������뻹ԭ������֮��Ϊ___________��
2K2S+K2SO3+3H2O���÷�Ӧ���������뻹ԭ������֮��Ϊ___________��
��3����������Ҫ��������ʣ��Լ�����_________��Ŀ����___________________________��
��4����������ʹ��ҺpH___________�������С������
��5�����������¶�Ϊ70 -80���Ŀ����_________________________________________ ��
��6��˫��ˮ�����Ի��������ᣩ����һ��ǿ�����������Խ������������������������Ƶ�Fe2(OH)n(SO4)3-n/2����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
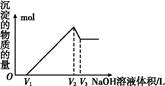
ij�о�С�齫һ����������·�徭Ũ�����ϡ���ᴦ����õ�һ�����Һ,���к���Cu2+��Fe2+��Fe3+��Al3+�Ƚ�������,��������������������Էֱ���ȡCuSO4��5H2O�����AlCl3
��Һ: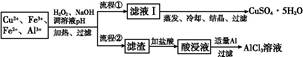
��֪:��ؽ������ӿ�ʼ��������ȫ����ʱ��pH��ΧΪ:
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com