
��������A�к���Ԫ��LiԪ�أ�A��Ħ������23g·mol-1��A��Ҫ�����л��ϳɺ�ҩ�����죬ͬʱҲ�����õĴ�����ϡ���һ�������£�0.1mol����A��0.1molNH4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L(��״��)����C����֪����C��������ˮ���ҵõ�������Һ�������ˮB�����ɽ�������D����������ش��������⣺
��1��A�Ļ�ѧ��ʽ��_________________________��
��2��д��������A��NH4Cl��Ӧ�Ļ�ѧ����ʽ:_______________________________��
��3��ijͬѧͨ���������ϵ�֪����A�����ʣ�
I.��ҵ�Ͽ��ý���D��Һ̬��C�����������·�Ӧ���Ʊ�A���ʡ�
II.����A��ˮǿ��ˮ�⣬�ͷų�����C��
��I�з�����Ӧ�Ļ�����Ӧ������__________________________��
������A��ˮǿ��ˮ��Ļ�ѧ����ʽΪ_________________________��
��4����ҵ�Ʊ�����D���������£�
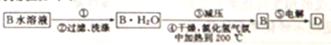
�ٲ�����в���������Ϊ________________��
������ƽ��ԭ�����Ͳ�����м�ѹ��Ŀ���ǣ�_________________________��
��֪ʶ�㡿������ƶϣ��Ʊ�ʵ�鷽������� C1 J5
���𰸽�����(1)LiNH2(2��) ��2��LiNH2��NH4Cl=LiCl��2NH3��
(3)���û���Ӧ(1��) ��LiNH2��2H2O=LiOH��NH3��(2��)
(4)������Ũ������ȴ�ᾧ LiCl·H2O(s) LiCl(s)��H2O(g)����Сѹǿ��������ƽ��������Ӧ�����ƶ����Ӷ���������ˮLiCl���Ʊ�����2�֣�
LiCl(s)��H2O(g)����Сѹǿ��������ƽ��������Ӧ�����ƶ����Ӷ���������ˮLiCl���Ʊ�����2�֣�
��������һ�������£�0.1mol����A��0.1molNH4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L(��״��)����C������C��������ˮ�õ�������Һ������֪CΪNH3�������ˮB������һ�ֶ�����Ԫ�صĽ�������D��������BΪ����D���Ȼ��4.48L���������ʵ���=4.48L/22.4L/mol=0.2mol��������=0.2mol��17g/mol=3.4g��0.1mol����A������Ϊ2.30g��0.1molNH4Cl���������Ϊ5.35g�����������غ��֪B������Ϊ2.3g+5.35g-3.4g=4.25g�� A�к�Li����DΪ��A������������A��NH4Cl���巴Ӧ�ɱ�Ϊ��A+NH4Cl��LiCl+NH3������Clԭ���غ㣬LiCl�����ʵ���=0.1mol����ô2.3g������A�к�LiԪ��ҲΪ 0.1mol���ٸ��������غ��ԭ���غ㣨ԭ�ӵ��������Ŀ��Ӧǰ����ͬ������2.3gA�к���Nԭ��Ϊ0.2mol-0.1mol=0.1mol������Hԭ��Ϊ0.2mol��4-0.4mol=0.2mol������֪A��LiNH2��
��1��������������֪��AΪLiNH2��CΪ�����������ʽΪ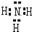 ��
��
��2��������A��NH4Cl��Ӧ�Ļ�ѧ����ʽΪLiNH2��NH4Cl=LiCl��2NH3��
��3���ٽ���Li��Һ̬��N3H�����������·�Ӧ���Ʊ�LiNH2����ͬʱ�������������ʷ�ӦΪ�û���Ӧ��������LiNH2��ˮ����ˮ�⣬Ӧ������ӽ��ˮ������������������ӣ�NH2—���ˮ��������������ӣ���ˮ�ⷴӦ����ʽΪ��LiNH2��2H2O=LiOH��NH3����
��4���������̿�֪Ӧ�Ǵ���Һ�еõ����壬������в�������Ϊ����Ũ������ȴ�ᾧ������LiCl﹒H2O⇌LiCl+H2O��֪��������м�ѹ��Ŀ���Ǽ�Сѹǿ������������ƽ�����������ƶ�����������ˮLiCl���Ʊ���
��˼·�㲦�����⿼�������ƶϡ���ѧʵ��ȣ���Ŀ�زıȽ�İ����������Ŀ�Ѷȣ����ؿ���ѧ����֪ʶ��Ǩ��Ӧ�����ۺϷ������������������ѧ�����������нϸߵ�Ҫ����A�к�LiԪ�أ������DΪLi�ǹؼ����ѶȽϴ�


 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)  ��������
��������
____________________________��
(2)2,4,6����5�һ�����ķ����й���______����ԭ���š�
(3)��������6������һ�����ֻ��1�ֵ������ķ���ʽ��__________����ṹ��ʽ��__________________��������______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں����ܱ�������ͨ��X��������Ӧ��2X(g) Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����
Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����
A���÷�Ӧ���е�M��ų����������ڽ��е�W��ų�������
B��T2�£���0��t1ʱ���ڣ�v(Y)��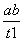 mol·L��1·min��1
mol·L��1·min��1
C��M�������Ӧ����v������N����淴Ӧ����v��
D��M��ʱ�ټ���һ����Y��ƽ���X���������ԭƽ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪AΪ����ɫ���壬T��RΪ���ֳ�������;�ܹ�Ľ������ʣ�D�Ǿ��д��Եĺ�ɫ���壬 C����ɫ��ζ�����壬H�ǰ�ɫ���������ڳ�ʪ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ���塣

��1��д���������ʵĻ�ѧʽ��
A�� D�� R�� ��
��2����Ҫ��д���з�Ӧ����ʽ��
H�ڳ�ʪ�����б��M�Ĺ����еĻ�ѧ����ʽ�� ��
��N��ͨ����CO2ʱ��Ӧ�����ӷ���ʽ�� ��
D�����ᷴӦ�����ӷ���ʽ�� ��
��3��������������C�ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ijЩ��ѧ֪ʶ��ͼ���ʾ�������յ�ֱ�ۡ�������Ч��������ͼ������ʾ�Ļ�ѧ֪ʶ�У�����ȷ���� �� ��
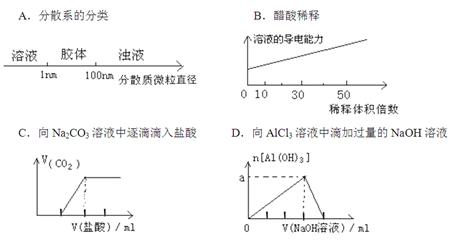
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������O2��CH4�Ļ�����������23.4g Na2O2���ܱ������У����ȼ����Ӧ��������������150��ʱѹǿԼΪ0��������������ˮ���������ݳ���
��1��ԭ���������O2��CH4�����Ϊ
��2����������ɷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڱ�����ŵĿհ״���ա�
��1������ȷ��ʾԭ�ӹ������____��____��
A.2s��������B.2d��������C.3px��������D.3f
��2��д����̬��(Ga)ԭ�ӵĵ����Ų�ʽ��____��____��
��3���������ʱ仯��ֻ�뷶�»����йص���__��____��
A.�ɱ��ۻ�
B.��������
C.�Ҵ����ͪ����
D.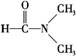 ����ˮ
����ˮ
E.���������Ȼ�̼
F.ʯӢ����
��4�����������У�ֻ���м��Լ��ķ�����____��____���Ⱥ����Ӽ��ֺ����ۼ��Ļ�������____��____��ֻ���ڦҼ��ķ�����____��____��ͬʱ���ڦҼ��ͦм��ķ�����____��____��
A.N2�� B.CO2�� C.CH2Cl2�� D.C2H4�� E.C2H6
F.CaCl2��G.NH4Cl
��5���á�>���� <����=����գ�
��һ�����ܵĴ�С��Mg____��____Al��
�۵�ĸߵͣ�KCl____��____MgO��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л������У������ϵ���ԭ�ӱ���ԭ��ȡ��������һ�����������ͬ���칹�����(����)
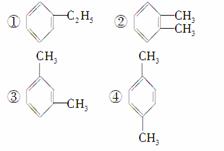
A���٢� B���٢�
C���ڢ� D���ۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com