
A��ƽ��ÿ����B����Ũ�ȵı仯Ϊ0.02p/tmol��L-1
B������ȷ��C���ʵ�״̬
C��x=3
D������A��Ũ�Ȼ������¶Ⱦ������A��ת����
 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
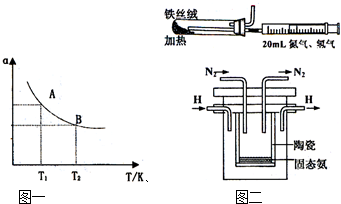 ��2009?��Զģ�⣩�¹��˹�����1909�귢���ĺϳɰ���Ӧԭ��Ϊ��N2��g��+3H2��g��?2NH3��g�� ��֪298Kʱ����H=-92.4kJ?mol-1�Իش��������⣺
��2009?��Զģ�⣩�¹��˹�����1909�귢���ĺϳɰ���Ӧԭ��Ϊ��N2��g��+3H2��g��?2NH3��g�� ��֪298Kʱ����H=-92.4kJ?mol-1�Իش��������⣺| 0.082 |
| 0.06��0.183 |
| 0.082 |
| 0.06��0.183 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 KH2PO4��aq��+HCl��aq��������Ӧʱ�䲻ͬʱ����Ʒ���ʺͲ�Ʒ��Cl?�����仯�����ͼ��ʾ��KDP�����һ��Ʒ����Cl-������������0.2%��
KH2PO4��aq��+HCl��aq��������Ӧʱ�䲻ͬʱ����Ʒ���ʺͲ�Ʒ��Cl?�����仯�����ͼ��ʾ��KDP�����һ��Ʒ����Cl-������������0.2%��

| 0.082 |
| 0.06��0.182 |
| 0.082 |
| 0.06��0.182 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com