
������A���緢���������У������������Ǵ�л���м��壬�����������������۵ȷ����Ƶá���ҵ��Ҳ���ɱ�ϩ�������з����ϳɣ�
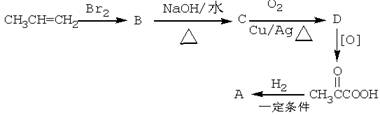
�ش��������⣺
��1��д�����з�Ӧ�ķ�Ӧ����ʽ����ע����Ӧ���ͣ�
 ��
��Ӧ���ͣ�
��
��Ӧ���ͣ�
 :
��Ӧ���ͣ�
��
:
��Ӧ���ͣ�
��
��2��A�Ľṹ��ʽΪ�� , Ũ������������������ӵ�A�ܷ�Ӧ������Ԫ��״�����д���û�����Ľṹ��ʽ: ��
��3��D�ĺ�������������Ϊ�� ��д��һ�ּ��ɣ�����֪D��һ��ͬ���칹���ܺ�NaHCO3��Һ��Ӧ�ų����壬д����ͬ���칹��Ľṹ��ʽ�� ��
��4�� ��һ�������������������ȡ���Ӧ������֮һΪ��ȩ��ͬϵ�д���ò���Ľṹ��ʽ��
��
��һ�������������������ȡ���Ӧ������֮һΪ��ȩ��ͬϵ�д���ò���Ľṹ��ʽ��
��
��1��CH3CHBrCH2Br +2NaOH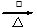 CH3CHOHCH2OH+2NaBr
2�� ȡ����Ӧ 1��
CH3CHOHCH2OH+2NaBr
2�� ȡ����Ӧ 1��
 2�� ������Ӧ 1��
2�� ������Ӧ 1��
��2�� 2��
2�� 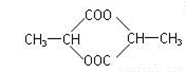 2��
2��
��3��ȩ�����ʻ� 2�� CH2=CHCOOH 2��
��4��CH3CHO 2��
��������
��������������л���ͼ��������Ϣ���Ƴ���CH3CH=CH2��Br2�ӳ����ɵ�BΪ��CH3CHBrCH2Br��B��NaOHˮ��Һ�з���ˮ�ⷴӦ���ɵ�CΪ��CH3CHOHCH2OH��C�е��ǻ��������õ���DΪ��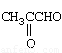 ���������H2�ļӳɷ�Ӧ����A���
���������H2�ļӳɷ�Ӧ����A���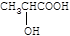 ��
��
��1��
 Ϊ���������NaOH��Һ�е�ˮ�ⷴӦ��CH3CHBrCH2Br +2NaOH
Ϊ���������NaOH��Һ�е�ˮ�ⷴӦ��CH3CHBrCH2Br +2NaOH CH3CHOHCH2OH+2NaBr����Ӧ����Ϊȡ����Ӧ��
CH3CHOHCH2OH+2NaBr����Ӧ����Ϊȡ����Ӧ�� Ϊ�������Ĵ�������
Ϊ�������Ĵ�������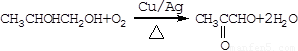 ����Ӧ����Ϊ������Ӧ��
����Ӧ����Ϊ������Ӧ��
��2��AΪ���ᣬ�ṹ��ʽΪ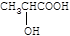 ��Ũ������������������ӵ�A�ܷ�Ӧ������Ԫ��״���������������Ӽ䷢��������Ӧ�����ɣ�
��Ũ������������������ӵ�A�ܷ�Ӧ������Ԫ��״���������������Ӽ䷢��������Ӧ�����ɣ�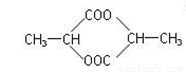 ��
��
��3��D�к���1���ʻ���1��ȩ�����ܺ�NaHCO3��Һ��Ӧ�ų����壬˵������ͬ���칹�庬���Ȼ������Ƴ��ṹ��ʽΪ�� CH2=CHCOOH��
��4��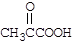 ��һ�������������������ȡ���Ӧ������֮һΪ��ȩ��ͬϵ�ʧȥCO2������CH3CHO��
��һ�������������������ȡ���Ӧ������֮һΪ��ȩ��ͬϵ�ʧȥCO2������CH3CHO��
���㣺���⿼���л��ϳɵķ�������ѧ����ʽ����д����Ӧ���͡�������ƶϡ�ͬ���칹����жϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�ϲ��и���������ģ��������ۻ�ѧ���� ���ͣ������
I��������Ա���֣�һЩ��ѧ��Ӧ�ڹ���֮�䷢������ˮ��Һ�з��������ﲻͬ��ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+1��+2����CuCl2��2H2O�����NaOH��������ĥ������������һ��ɫ�Ĺ���M��M������ˮ����������ϡ����������ɫ��ҺB��
M�Ļ�ѧʽΪ ��M��ͬ��CuCl2��NaOH����Һ�з�Ӧ���ò���Ŀ���ԭ����
��
II��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ������ѧ������Ӧ�Ļ�ѧ����ʽ������A��B�����ʵ���֮��Ϊ1��4����ش�
��1����Y�ǻ���ɫ���壬��Y�ĵ���ʽ�� ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��2����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ��
��AԪ�������ڱ��е�λ���� �����������ں��壩��Y�Ļ�ѧʽ�� ��
�ں�a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X��____mol��
��3����A��B��X��Y��Ϊ�����A����ˮ������������Ӻ�ˮ���õ�������ɾ���ˮ����A��Һ�м��������ữ��AgNO33��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1��4ǡ�÷�Ӧ����Һ������Ũ�ȴӴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��Ц����(N2O)����������Ӧ����ҽ�Ƶ�������֮һ���й�������Ϊ��N2O��CO2���Ӿ������ƵĽṹ(��������ʽ)����֪N2O��������ԭ��ֻ��һ����ԭ����������N2O�ĵ���ʽ�ɱ�ʾΪ__________����ռ乹����__________�ͣ��ɴ˿�֪����__________����(����ԡ��Ǽ��ԡ�)��
(2)��һ�ֳ����������ȷ£����治�����������������綾�����(COCl2)��2CHCl3+O2 ![]() 2HCl+2COCl2��Ϊ��ֹ�¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ���ʵ��Լ���___________��
2HCl+2COCl2��Ϊ��ֹ�¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ���ʵ��Լ���___________��
A.���۵⻯����Һ B.NaOH��Һ
C.��̪��Һ D.�����ữ����������Һ
(3)��Ϊ�������������ҽѧ�Ϻ������ӡ��������ϸ���ʵ���֬�����ϸ�������������ͣ��Ӷ�ʹ��ĩ��������ʱֹͣ�������������������Ϊ80%�������20��������ɵĻ�����壬��Ϊ�����õ���������
���Ԫ�����ڱ���λ��_________���ڣ�__________�壬����ԭ������Ϊ____________��
��Ϊϡ������(��ƶ�������)��믵Ļ�ѧ���ʲ����ã�������ȷ����һ�����������ɻ������ȡ1 mol�����3.5 mol�������ܱ������У�����������1.5 mol����ͬʱ�а�ɫ�����γɣ��˰�ɫ����Ļ�ѧʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I��������Ա���֣�һЩ��ѧ��Ӧ�ڹ���֮�䷢������ˮ��Һ�з��������ﲻͬ��ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+1��+2����CuCl2��2H2O�����NaOH��������ĥ������������һ��ɫ�Ĺ���M��M������ˮ����������ϡ����������ɫ��ҺB��
M�Ļ�ѧʽΪ ��M��ͬ��CuCl2��NaOH����Һ�з�Ӧ���ò���Ŀ���ԭ����
��
II��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ������ѧ������Ӧ�Ļ�ѧ����ʽ������A��B�����ʵ���֮��Ϊ1��4����ش�
��1����Y�ǻ���ɫ���壬��Y�ĵ���ʽ�� ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��2����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ��
��AԪ�������ڱ��е�λ���� �����������ں��壩��Y�Ļ�ѧʽ�� ��
�ں�a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X��____mol��
��3����A��B��X��Y��Ϊ�����A����ˮ������������Ӻ�ˮ���õ�������ɾ���ˮ����A��Һ�м��������ữ��AgNO33��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1��4ǡ�÷�Ӧ����Һ������Ũ�ȴӴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ�ϲ��и���������ģ��������ۻ�ѧ���� ���ͣ������
I�� ������Ա���֣�һЩ��ѧ��Ӧ�ڹ���֮�䷢������ˮ��Һ�з��������ﲻͬ��ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij�����
������Ա���֣�һЩ��ѧ��Ӧ�ڹ���֮�䷢������ˮ��Һ�з��������ﲻͬ��ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij����� �ϼ���+1��+2����CuCl2��2H2O�����NaOH��������ĥ����
�ϼ���+1��+2����CuCl2��2H2O�����NaOH��������ĥ���� ��������һ��ɫ�Ĺ���M��M������ˮ����������ϡ����������ɫ��ҺB��
��������һ��ɫ�Ĺ���M��M������ˮ����������ϡ����������ɫ��ҺB��
M�Ļ�ѧʽΪ ��M��ͬ��CuCl2��NaOH����Һ�з�Ӧ���ò���Ŀ���ԭ����
��
II��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ������ѧ������Ӧ�Ļ�ѧ����ʽ������A��B�����ʵ���֮��Ϊ1��4����ش�
��1����Y�ǻ���ɫ���� ����Y�ĵ���ʽ�� ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
����Y�ĵ���ʽ�� ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��2����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ��
��AԪ�������ڱ��е�λ���� �����������ں��壩��Y�Ļ�ѧʽ�� ��
�ں�a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X��____mol��
��3����A��B��X��Y��Ϊ�����A�� ��ˮ������������Ӻ�ˮ���õ�������ɾ���ˮ����A��Һ�м��������ữ��AgNO33��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1��4ǡ�÷�Ӧ����Һ������Ũ�ȴӴ�С��˳���� ��
��ˮ������������Ӻ�ˮ���õ�������ɾ���ˮ����A��Һ�м��������ữ��AgNO33��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1��4ǡ�÷�Ӧ����Һ������Ũ�ȴӴ�С��˳���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com