
 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²�������� 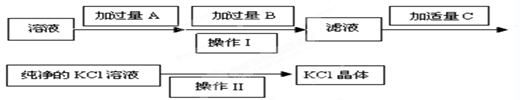
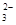 ��OH����HCO
��OH����HCO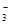 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����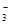 �ų���������ϡ�����ữ�������������˵������CO
�ų���������ϡ�����ữ�������������˵������CO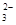 ������ԭ��Һ��һ�����ڵ�������Na+����Һ�����ٺ���һ�������ӣ�,CO32��,OH����һ�������ڵ�������Fe3+,H+,Mg2+,HCO3���������ܿ϶��Ƿ���ڵ�������Cl����ȡ����ԭ��Һ����������ϡ�����ữ���ٵμ�����AgNO3,���а�ɫ��������������Cl��,����û�С�
������ԭ��Һ��һ�����ڵ�������Na+����Һ�����ٺ���һ�������ӣ�,CO32��,OH����һ�������ڵ�������Fe3+,H+,Mg2+,HCO3���������ܿ϶��Ƿ���ڵ�������Cl����ȡ����ԭ��Һ����������ϡ�����ữ���ٵμ�����AgNO3,���а�ɫ��������������Cl��,����û�С�

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��������һ����CO32�� |
| B������KSCN��Һ�������������ٵμӼ���������ˮ����Һ��ΪѪ��ɫ����������һ����Fe2+ |
| C������BaCl2��Һ��������ɫ�������ټ��������ϡ�����ữ���������ܽ⣬��������һ����SO42�� |
| D���ھƾ��ƻ��������գ�����Ϊ��ɫ����������һ������Ԫ�أ�һ��������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
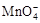 ����C2O42?�����ӷ���ʽ����������
����C2O42?�����ӷ���ʽ����������| ����KMnO4��Һ�Ĵ��� | KMnO4��Һ��ɫ��ȥ�����ʱ�� |
| �ȵ����1�� | 60 s |
| ��ɫ���ٵ����2�� | 15 s |
| ��ɫ���ٵ����3�� | 3 s |
| ��ɫ���ٵ����4�� | 1 s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����Ba��NO3��2��Һ��ͨ��SO2���������� |
| B����Na2SiO3��Һ��ͨ��HCl���������� |
| C�������ʯ��ˮ��ͨ��CO2���������� |
| D����NaAlO2��Һ��ͨ��HCl���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H+��NO3����Fe2+��Na+������������Һ���ܹ��������� |
| B��Cl2ͨ��ˮ�е����ӷ���ʽ��Cl2+H2O��2H++Cl��+ClO�� |
| C��1mol Na2O2�к���2mol�����Ӻ�1mol������ |
| D����ij��Һ�м������ʯ��ˮ����ǣ��ټ������ᣬ�����������ɫ��ζ�����������ԭ��Һ��һ������̼������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
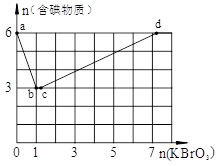
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��NaHSO4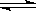 Na++H++SO42һ Na++H++SO42һ | B��NaHCO3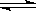 Na++ HCO3һ Na++ HCO3һ |
C��H2S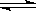 2H++S2������ 2H++S2������ | D��Mg(OH)2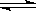 Mg2++2OH- Mg2++2OH- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com