
 �ⶨ������������
�ⶨ������������ �ⶨ������������
�ⶨ������������ ��Һ
��Һ ����Һ
����Һ �������ղ����������
�������ղ����������
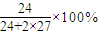 =30.8%���ʴ�Ϊ���ܣ�30.8%��
=30.8%���ʴ�Ϊ���ܣ�30.8%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����| NaOH��Һ |
| ���� |
| ���� |
| ����NaOH��Һ |
| ���ˡ�ϴ�ӡ����ơ���ȴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�Ͳ�һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� ��Һ
��Һ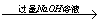 ����Һ
����Һ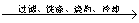 �������ղ����������
�������ղ����������
(1)д������һ�з�����Ӧ�����ӷ���ʽ ��
(2)ʵ��С����ݷ��������������ʵ��װ�ã�����Ȧ(ͼ�е�����̨��ʡ��)��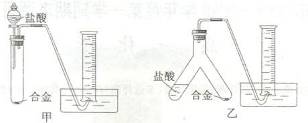
����Ϊѡ�� ��ѡ�����)װ�ý���ʵ�������������С��
(3)�÷���������ʵ��ʱ�����˳������ղ��������⣬��������� ��
(4)��չ�о���������þ�Ͻ�������������Һ�м������ ��Һʱ�����ɳ��������������
��Һʱ�����ɳ�������������� ��Һ����Ĺ�ϵ���������ϵ��ʾ��
��Һ����Ĺ�ϵ���������ϵ��ʾ��
�����жϣ�������ͼ������������ܷ�����Ͻ���þ����������? (ѡ��ܡ����ܡ�)_____________.
���Т٢�����ѡһ������
������������Ͻ���þ��������������˵������ ��
����������Ͻ���þ��������������þ����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ͼ�ѧ�����ר��ѧУ��һ���ڽ�ҵ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ�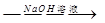 �ⶨ������������
�ⶨ������������
����������þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� ��Һ
��Һ
 �������ղ����������
�������ղ����������
(1)ʵ��С����ݷ��������������ʵ��װ�ã�����Ȧ(ͼ�е�����̨��ʡ��)��
����Ϊѡ�� ��ѡ�����)װ�ý���ʵ�������������С��
(2)�÷���������ʵ��ʱ�����˳������ղ��������⣬����������� ��
(3)��չ�о���������þ�Ͻ�������������Һ�м������ ��Һʱ�����ɳ��������������
��Һʱ�����ɳ�������������� ��Һ����Ĺ�ϵ���������ϵ��ʾ��
��Һ����Ĺ�ϵ���������ϵ��ʾ��
�����жϣ�������ͼ������������ܷ�����Ͻ���þ����������? (ѡ��ܡ����ܡ�)
���Т٢�����ѡһ������
������������Ͻ���þ��������������˵������ ��
����������Ͻ���þ��������������þ����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� ��Һ
��Һ ����Һ
����Һ �������ղ����������
�������ղ����������
(1)д������һ�з�����Ӧ�����ӷ���ʽ ��
(2)ʵ��С����ݷ��������������ʵ��װ�ã�����Ȧ(ͼ�е�����̨��ʡ��)��
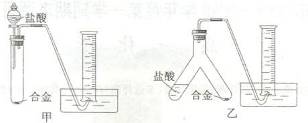
����Ϊѡ�� ��ѡ�����)װ�ý���ʵ�������������С��
(3)�÷���������ʵ��ʱ�����˳������ղ��������⣬��������� ��
(4)��չ�о���������þ�Ͻ�������������Һ�м������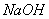 ��Һʱ�����ɳ��������������
��Һʱ�����ɳ��������������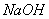 ��Һ����Ĺ�ϵ���������ϵ��ʾ��
��Һ����Ĺ�ϵ���������ϵ��ʾ��
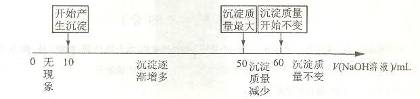
�����жϣ�������ͼ������������ܷ�����Ͻ���þ����������? (ѡ��ܡ����ܡ�)_____________.
���Т٢�����ѡһ������
������������Ͻ���þ��������������˵������ ��
����������Ͻ���þ��������������þ����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ͼ�ѧ�����ר��ѧУ��һ���ڽ�ҵ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ�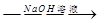 �ⶨ������������
�ⶨ������������
����������þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� ��Һ
��Һ
 �������ղ����������
�������ղ����������
(1)ʵ��С����ݷ��������������ʵ��װ�ã�����Ȧ(ͼ�е�����̨��ʡ��)��
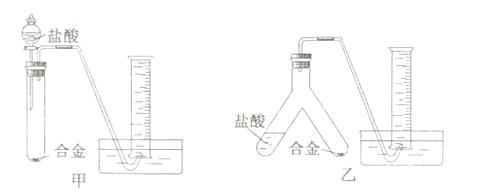
����Ϊѡ�� ��ѡ�����)װ�ý���ʵ�������������С��
(2)�÷���������ʵ��ʱ�����˳������ղ��������⣬����������� ��
(3)��չ�о���������þ�Ͻ�������������Һ�м������ ��Һʱ�����ɳ��������������
��Һʱ�����ɳ�������������� ��Һ����Ĺ�ϵ���������ϵ��ʾ��
��Һ����Ĺ�ϵ���������ϵ��ʾ��
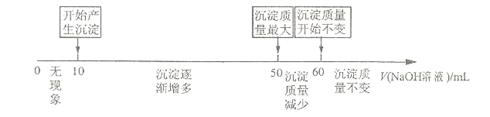
�����жϣ�������ͼ������������ܷ�����Ͻ���þ����������? (ѡ��ܡ����ܡ�)
���Т٢�����ѡһ������
������������Ͻ���þ��������������˵������ ��
����������Ͻ���þ��������������þ����������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com