
��14�֣�J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����±���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к�������Ԫ�ء�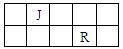
��1��Ԫ��T��������
��2��J����Ԫ����ɵĻ�����Aÿ��������4��ԭ����ɣ���֪���ȼ��a gA����ʱ����1mol������̼�����Һ̬ˮ�����ų�����b kJ����A����ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����ҵȼ�ս�̿���β���к���M��R��ɵ����壬Ϊ�˲ⶨ�京��������ѡ����Լ���
| A������������Һ | B�������ữ�ĸ��������Һ | C�����۵⻯����Һ | D����ˮ��Һ |
 ���ּ�̬��ͬ�����廯��������ת������ͬ��ͬ�ݵļ��������зֱ����1mol��2mol��LM2���壬�ֱ���ƽ�⣬����������LM2��ת���ʱȽϼ� �ң�<��>��=��
���ּ�̬��ͬ�����廯��������ת������ͬ��ͬ�ݵļ��������зֱ����1mol��2mol��LM2���壬�ֱ���ƽ�⣬����������LM2��ת���ʱȽϼ� �ң�<��>��=��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�

 Al��OH��3+3HCl
Al��OH��3+3HCl Al��OH��3+3HCl
Al��OH��3+3HCl| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����N2�����ʵ��� | ��������ʵ��� |
| y | �����ʵ��� | ƽ�ⳣ��K | ��ת���� | ���������ʵ����ܺ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
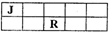 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| J | ||||
| M | R |

| ѡ�� | a | b | c | d |
| x | �¶� | ����H2�����ʵ��� | ����H2�����ʵ��� | ��������ʵ��� |
| y | �����ʵ��� | ƽ�ⳣ��K | ��ת���� | ���������ʵ����ܺ� |
| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com