
| A�� | V=336 mLʱ��c��OH-��=c��H+��+c��HCO3-��+2c��H2CO3�� | |
| B�� | V=448 mLʱ��2c��Na+��=3[c��H2CO3��+c��HCO3-��+c��CO32-��] | |
| C�� | V=672 mLʱ��c��Na+����c��HCO3-����c��OH-����c��CO32-����c��H+�� | |
| D�� | ͨ��CO2�����к��й�ϵʽ��c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-�� |
���� ���ܷ����ķ�Ӧ��CO2+2NaOH=Na2CO3+H2O��CO2+NaOH=NaHCO3��NaOH�����ʵ���Ϊ0.2L��0.15mol/L=0.03mol��
A��������̼Ϊ336mLʱ�������ʵ���Ϊ$\frac{0.336L}{22.4L/mol}$=0.015mol��n��NaOH����n��CO2��=2��1����Ӧ�õ�Na2CO3��Һ��������Һ�������������غ��жϣ�
B��������̼Ϊ448mLʱ�������ʵ���Ϊ$\frac{0.448L}{22.4L/mol}$=0.02mol��1��n��NaOH����n��CO2��=3��2��2����Ӧ�õ�Na2CO3��NaHCO3�����Һ����������ʵ����ֱ�Ϊxmol��ymol����$\left\{\begin{array}{l}{x+y=0.02}\\{2x+y=0.03}\end{array}\right.$�����x=y=0.01�����������غ��жϣ�
C��������̼Ϊ672mLʱ�������ʵ���Ϊ$\frac{0.672L}{22.4L/mol}$=0.03mol��n��NaOH����n��CO2��=1��1����Ӧ�õ�NaHCO3��Һ����Һ��̼���������ˮ�⣬��Һ�ʼ��ԣ���Һ��ˮҲ����������������ӣ�
D����Һ�ʵ����ԣ����ݵ���غ��жϣ�
��� �⣺���ܷ����ķ�Ӧ��CO2+2NaOH=Na2CO3+H2O��CO2+NaOH=NaHCO3��NaOH�����ʵ���Ϊ0.2L��0.15mol/L=0.03mol��
A��������̼Ϊ336mLʱ�������ʵ���Ϊ$\frac{0.336L}{22.4L/mol}$=0.015mol��n��NaOH����n��CO2��=2��1����Ӧ�õ�Na2CO3��Һ����Һ��̼�������ˮ�⣬������Һ�������������غ㣺c��OH-��=c��H+��+c��HCO3-��+2c��H2CO3������A��ȷ��
B��������̼Ϊ448mLʱ�������ʵ���Ϊ$\frac{0.448L}{22.4L/mol}$=0.02mol��1��n��NaOH����n��CO2��=3��2��2����Ӧ�õ�Na2CO3��NaHCO3�����Һ����������ʵ����ֱ�Ϊxmol��ymol����$\left\{\begin{array}{l}{x+y=0.02}\\{2x+y=0.03}\end{array}\right.$�����x=y=0.01���������غ��֪��2c��Na+��=3[c��H2CO3��+c��HCO3-��+c��CO32-��]����B��ȷ��
C��������̼Ϊ672mLʱ�������ʵ���Ϊ$\frac{0.672L}{22.4L/mol}$=0.03mol��n��NaOH����n��CO2��=1��1����Ӧ�õ�NaHCO3��Һ����Һ��̼���������ˮ�⣬��Һ�ʼ��ԣ���Һ��ˮҲ����������������ӣ���Һ������Ũ��Ϊc��Na+����c��HCO3-����c��OH-����c��H+����c��CO32-������C����
D����Һ�ʵ����ԣ����ݵ���غ㣬ͨ��CO2�����к��й�ϵʽ��c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-������D��ȷ��
��ѡ��C��
���� ���⿼������Ũ�ȴ�С�Ƚϣ����Ϸ�����Ӧ���жϷ�Ӧ������������ٽ��������ʵ��롢����ˮ�⡢ˮ�ĵ����Լ�����غ㡢���غ㡢���Ӻ��ʽ���з����жϣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������һ�����õĵ��ȡ�������� | |
| B�� | Ԫ�صķǽ�����Խǿ�����⻯��ķе�Խ�� | |
| C�� | ͬ������������Ԫ�ص�ԭ�ӣ���������֮���Ϊ1 | |
| D�� | ����������Ϊ4��ԭ�ӣ���Ԫ��һ�����ڢ�A�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ϡ�������ά�ͺϳ��������л��߷��ӻ����� | |
| B�� | ʳƷ���������ʢ�й轺�����۵�С�����ɷ�ֹʳ���ܳ����������� | |
| C�� | Na2FeO4����ˮ������Ӧ����Fe��OH��3��O2��������Ϊ����ˮ���������;����� | |
| D�� | ��ľ�Һ��̬���ʲ��ܻ��ʹ�ã�����ΪNH4++HCO3-�TCO2��+H2O+NH3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��1s22s22p6 | B�� | Br��1s22s22p63s23p63d104s24p5 | ||
| C�� | O2-��1s22s22p6 | D�� | Cr��ls22s22p63s23p63d44s2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
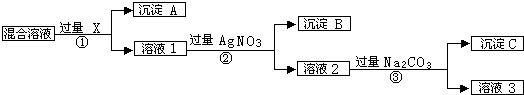
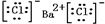 ������A�ľ����������Ӿ��壬CO32-�Ŀռ乹����ƽ�������Σ�
������A�ľ����������Ӿ��壬CO32-�Ŀռ乹����ƽ�������Σ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢۢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
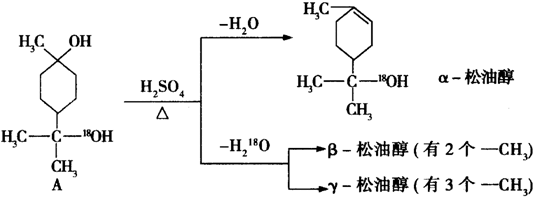
 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$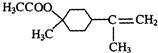 +H2O��
+H2O��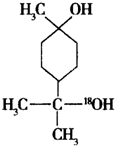 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$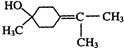 +H218O���÷�Ӧ�ķ�Ӧ��������ȥ��Ӧ��
+H218O���÷�Ӧ�ķ�Ӧ��������ȥ��Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��A2C2�Ľṹʽ��H-O-O-H��
��A2C2�Ľṹʽ��H-O-O-H�� ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com