
ЩшNAДњБэАЂЗќМгЕТТоГЃЪ§ЕФЪ§жЕЃЌЯТСаЫЕЗЈжае§ШЗЕФЪЧ ( )
ЂйГЃЮТГЃбЙЯТЃЌ17gМзЛљ(ЁЊ14CH3)ЫљКЌЕФжазгЪ§ЮЊ9NA
ЂкГЃЮТГЃбЙЯТЃЌ22.4 L NOЦјЬхЕФЗжзгЪ§аЁгкNA
Ђл64 gЭЗЂЩњбѕЛЏЛЙдЗДгІЃЌвЛЖЈЪЇШЅ2NAИіЕчзг
ЂмГЃЮТГЃбЙЯТЃЌ100 mL 0.5 molЁЄLЃ1ЕФввЫсШмвКжаЃЌввЫсЕФЗжзгЪ§ФПаЁгк0.05NA
ЂнБъзМзДПіЯТЃЌ22.4 LЖўТШМзЭщЫљКЌгаЕФЗжзгЪ§ЮЊNA
ЂоГЃЮТГЃбЙЯТЃЌ1 molКЄЦјКЌгаЕФКЫЭтЕчзгЪ§ЮЊNA
AЃЎЂйЂк BЃЎЂлЂм CЃЎЂкЂм DЃЎЂнЂо
C
ЁОНтЮіЁП1 molЁЊ14CH3жаЫљКЌжазгЕФЮяжЪЕФСПЮЊ(14Ѓ6) molЃЋ(1Ѓ1)ЁС3 molЃН8 mol,17 gЁЊ14CH3МДЮЊ1 molЃЌЂйВЛе§ШЗЃЛГЃЮТГЃбЙЯТЃЌ22.4 L NOЦјЬхЕФЮяжЪЕФСПаЁгк1 molЃЌдђЂке§ШЗЃЛЭЗЂЩњбѕЛЏЛЙдЗДгІЪБПЩЩњГЩЃЋ1МлЛђЃЋ2МлСНжжЛЏКЯЮяЃЌЫљвд1 mol CuПЩЪЇШЅ1 molЛђ2 molЕчзгЃЌЂлВЛе§ШЗЃЛгЩгкCH3COOHЮЊШѕЕчНтжЪЃЌПЩЗЂЩњЕчРыЃЌдђЂме§ШЗЃЛCH2Cl2дкБъзМзДПіЯТЮЊвКЬхЃЌЂнВЛе§ШЗЃЛКЄЦјЮЊЕЅдзгЗжзгЃЌ1 mol HeКЌга2 molЕчзгЃЌЂоВЛе§ШЗЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцзмИДЯА 10-3 ГЃМћЦјЬхЕФжЦБИСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
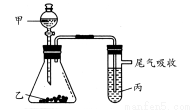
гУЯТЭМЫљЪОзАжУНјааЪЕбщЃЌЯТБэжаЪЕбщНсЙћФмЫЕУїНсТлЕФЪЧ( )
МзввБћНсТл
AЫЎЕчЪЏфхЫЎЮШЖЈадЃКЫЎЃОC2H2ЃОBr2
BбЮЫсЪЏЛвЪЏБНЗгФЦШмвКЫсадЃКHClЃОH2CO3ЃОБНЗг
CбЮЫсFeSфхЫЎЛЙдадЃКS2ЃЃОBrЃЃОClЃ
DХЈбЮЫсKMnO4KBrШмвКбѕЛЏадЃКKMnO4ЃМCl2ЃМBr2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцзмИДЯА 1-3 ШмвКЕФХфжЦМАЗжЮіСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
вбжЊСђЫсЁЂАБЫЎЕФУмЖШгыЫљМгЫЎСПЕФЙиЯЕШчЭМЫљЪОЃЌЯжгаСђЫсгыАБЫЎИївЛЗнЃЌЧыИљОнБэжааХЯЂЃЌЛиД№ЯТСаЮЪЬтЃК

| ШмжЪЕФЮяжЪЕФСП ХЈЖШ(molЁЄLЃ1) | ШмвКЕФУмЖШ(gЁЄcmЃ3) |
СђЫс | c1 | Іб1 |
АБЫЎ | c2 | Іб2 |
(1)БэжаСђЫсЕФжЪСПЗжЪ§ЮЊ (ВЛаДЕЅЮЛЃЌгУКЌc1ЁЂІб1ЕФДњЪ§ЪНБэЪО)ЁЃ
(2)ЮяжЪЕФСПХЈЖШЮЊc1 molЁЄLЃ1ЕФСђЫсгыЫЎЕШЬхЛ§ЛьКЯ(ЛьКЯКѓШмвКЬхЛ§БфЛЏКіТдВЛМЦ)ЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШЮЊ molЁЄLЃ1ЁЃ
(3)ЮяжЪЕФСПХЈЖШЮЊc2 molЁЄLЃ1ЕФАБЫЎгы c2 molЁЄLЃ1ЕФАБЫЎЕШжЪСПЛьКЯЃЌЫљЕУШмвКЕФУмЖШ (ЬюЁАДѓгкЁБЁЂЁАаЁгкЁБЛђЁАЕШгкЁБЃЌЯТЭЌ)Іб2 gЁЄcmЃ3ЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШ
c2 molЁЄLЃ1ЕФАБЫЎЕШжЪСПЛьКЯЃЌЫљЕУШмвКЕФУмЖШ (ЬюЁАДѓгкЁБЁЂЁАаЁгкЁБЛђЁАЕШгкЁБЃЌЯТЭЌ)Іб2 gЁЄcmЃ3ЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШ  c2 molЁЄLЃ1(ЩшЛьКЯКѓШмвКЕФЬхЛ§БфЛЏКіТдВЛМЦ)ЁЃ
c2 molЁЄLЃ1(ЩшЛьКЯКѓШмвКЕФЬхЛ§БфЛЏКіТдВЛМЦ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцзмИДЯА 1-2 ЮяжЪЕФСПЁЂЮяжЪЕФОлМЏзДЬЌСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
(1)дкЭЌЮТЁЂЭЌбЙЯТЃЌЪЕбщВтЕУCOЁЂN2КЭO2Ш§жжЦјЬхЕФЛьКЯЦјЬхЕФУмЖШЪЧH2ЕФ14.5БЖЃЌЦфжаO2ЕФжЪСПЗжЪ§ЮЊ ЁЃШєЦфжаCOКЭN2ЕФЮяжЪЕФСПжЎБШЮЊ1ЃК1ЃЌдђЛьКЯЦјЬхжабѕдЊЫиЕФжЪСПЗжЪ§ЮЊ ЁЃ
(2)ЯрЭЌЬѕМўЯТЃЌФГCl2гыO2ЛьКЯЦјЬх100 mLЧЁКУгы150 mL H2ЛЏКЯЩњГЩHClКЭH2OЃЌдђЛьКЯЦјЬхжаCl2гыO2ЕФЬхЛ§БШЮЊ ЃЌЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПЮЊ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцзмИДЯА 1-2 ЮяжЪЕФСПЁЂЮяжЪЕФОлМЏзДЬЌСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
вбжЊQгыRЕФФІЖћжЪСПжЎБШЮЊ9ЃК22ЃЌдкЗДгІXЃЋ2Y=2QЃЋRжаЃЌЕБ1.6 g XКЭYЭъШЋЗДгІКѓЩњГЩ4.4 g RЃЌдђВЮгыЗДгІЕФYКЭЩњГЩЮяQЕФжЪСПжЎБШЮЊ( )
AЃЎ46ЃК9 BЃЎ32ЃК9 CЃЎ23ЃК9 DЃЎ16ЃК9
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцзмИДЯА 1-1 ЮяжЪЕФЗжРрЁЂзЊЛЏМАЗжЩЂЯЕСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
вдЯТЪЧвРОнвЛЖЈЕФЗжРрБъзМЃЌЖдФГаЉЮяжЪгыЫЎЗДгІЧщПіНјааЗжРрЕФЗжРрЭМЁЃЧыИљОнФуЫљбЇЕФжЊЪЖЃЌАДвЊЧѓЬюПеЃК
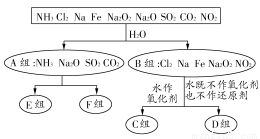
(1)ЩЯЪіЕквЛМЖЗжРрБъзМ(ЗжГЩAЁЂBзщЫљвРОн)ЪЧ ЁЃ
(2)CзщЮяжЪЮЊ ЁЃ
(3)DзщЮяжЪжаЃЌгыЫЎЗДгІЪБбѕЛЏМСКЭЛЙдМСЕФЮяжЪЕФСПжЎБШЮЊ1ЃК1ЕФЮяжЪЪЧ (ЬюЛЏбЇЪН)ЁЃ
(4)ШєEзщКЭFзщОљгаСНжжЮяжЪЃЌдђЦфЗжРрвРОнЪЧ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцзмИДЯА 1-1 ЮяжЪЕФЗжРрЁЂзЊЛЏМАЗжЩЂЯЕСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЗжРрЗНЗЈдкЛЏбЇбЇПЦЕФЗЂеЙжаЦ№ЕНживЊЕФзїгУЁЃЯТСаЗжРрБъзМКЯРэЕФЪЧ ( )
AЃЎИљОнбѕЛЏЮяЕФдЊЫизщГЩЃЌНЋбѕЛЏЮяЗжЮЊЫсадбѕЛЏЮяКЭМюадбѕЛЏЮя
BЃЎИљОнШмвКЕМЕчФмСІЕФЧПШѕЃЌНЋЕчНтжЪЗжЮЊЧПЕчНтжЪКЭШѕЕчНтжЪ
CЃЎИљОнЪЧЗёОпгаЖЁДяЖћаЇгІЃЌНЋЗжЩЂЯЕЗжЮЊШмвККЭНКЬх
DЃЎИљОнЛЏбЇЗДгІжаЮяжЪьЪжЕЕФБфЛЏЃЌНЋЛЏбЇЗДгІЗжЮЊЗХШШЗДгІКЭЮќШШЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцвЛТжИДЯА3-2ДгТСЭСПѓЕНТСКЯН№СЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
AЁЂBЁЂCЁЂDЁЂEЮхжжЛЏКЯЮяОљКЌгаФГжжЖЬжмЦкГЃМћдЊЫиЃЌЫќУЧЕФзЊЛЏЙиЯЕШчЭМЫљЪОЃЌЦфжаAЕФШмвКЮЊГЮЧхШмвКЃЌCЮЊФбШмЕФАзЩЋЙЬЬхЃЌEдђвзШмгкЫЎЃЌШЁAЕФШмвКзЦЩеЃЌбцЩЋЗДгІЮЊЧГзЯЩЋ(ЭИЙ§РЖЩЋюмВЃСЇ)ЁЃ
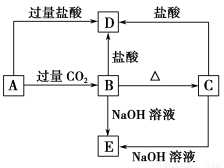
(1)аДГіЛЏбЇЪНЃКA ЃЌB ЃЌC ЃЌD ЃЌE ЁЃ
(2)аДГіЯТСаЗДгІЕФРызгЗНГЬЪНЃК
AЁњBЃК ЃЛ
AЁњDЃК ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЫеНЬАцвЛТжИДЯА2-2ЕчНтжЪ РызгЗДгІСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаа№Ъіжае§ШЗЕФЪЧ (ЁЁЁЁ)ЁЃ
AЃЎЮяжЪЕФШмНтЙ§ГЬЃЌЪЕжЪЩЯОЭЪЧЦфЕчРыЙ§ГЬ
BЃЎШ§бѕЛЏСђЕФЫЎШмвКФмЕМЕчЃЌЫљвдШ§бѕЛЏСђЪЧЕчНтжЪ
CЃЎ1 L 0.1 molЁЄLЃ1ЕФH2SO4ШмвКжаКЌга0.2 mol HЃЋ
DЃЎ1 L 0.1 molЁЄLЃ1ЕФH2SO3ШмвКжаКЌга0.2 mol HЃЋ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com