
(7��)�ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
(1)��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壮
��D�Ļ�ѧʽ��________��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ���________����Ⱦ�˻�����
(2)��A�ڳ�����Ϊ���壬C�Ǻ���ɫ�����壮
��A��C�Ļ�ѧʽ�ֱ��ǣ�A________��C________.
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ___________________________.�÷�Ӧ________(����ڡ������ڡ�)������ԭ��Ӧ��
(1)��H2SO4�� ������
(2)��NH3��N2��NO2 ��Cu+4HNO3(Ũ)=Cu(NO3)2+2NO2��+2H2O ����
��������������ӿ�ͼ�п��Կ����������������⡣��1��B����ʹƷ����ɫ���д̼�����ζ����ɫ���壬��B��SO2��DΪH2SO4��SO2���γ��������Ҫ���塣��2��C�Ǻ���ɫ�����壬��C��NO2��A��NH3��N2��D��HNO3��ͭ��Ũ���ᷴӦ�ķ���ʽΪ��Cu+4HNO3(Ũ)= Cu(NO3)2 +2NO2��+2H2O������������ԭ��Ӧ��
���㣺Ԫ���ƶ�
�����������������������ƶϣ��ܷ������������������е��ʣ�Na��C��S��N�������NH3��H2S��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
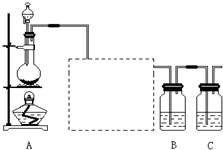 ��֪�ǽ���������S���ǵ���ɫ�����ĩ��������ˮ��Ϊ����֤��Ԫ�صķǽ����Ա���Ԫ�صķǽ�����ǿ��ij��ѧʵ��С�����������ʵ�飬��ش��������⣺
��֪�ǽ���������S���ǵ���ɫ�����ĩ��������ˮ��Ϊ����֤��Ԫ�صķǽ����Ա���Ԫ�صķǽ�����ǿ��ij��ѧʵ��С�����������ʵ�飬��ش��������⣺

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����������¿���ѧ�Ծ��������棩 ���ͣ������
(7��)�ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺

(1)��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壮
��D�Ļ�ѧʽ��________��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ���________����Ⱦ�˻�����
(2)��A�ڳ�����Ϊ���壬C�Ǻ���ɫ�����壮
��A��C�Ļ�ѧʽ�ֱ��ǣ�A________��C________.
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ___________________________.�÷�Ӧ________(����ڡ������ڡ�)������ԭ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧ����һ������ �ǽ������仯������ϰ���������棩 ���ͣ������
(7��)�ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
(1)��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壮
��D�Ļ�ѧʽ��________��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ���________����Ⱦ�˻�����
(2)��A�ڳ�����Ϊ���壬C�Ǻ���ɫ�����壮
��A��C�Ļ�ѧʽ�ֱ��ǣ�A________��C________.
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________________________________________________________.�÷�Ӧ________(����ڡ������ڡ�)������ԭ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ09-10ѧ���һ��ѧ��4���¿� ���ͣ������
��ÿ��3�֣���15�֣���֪����Ԫ��A��B��C��D����ԭ��������������������������Ϊ4��1��2��7������AԪ��ԭ�Ӵ���������Ϊ2��B��C ��Ԫ�ص�ԭ�Ӵ���������Ϊ8��BԪ��ԭ�������������ȵ�1���������1��Ҳ��CԪ��ԭ�ӵ�M���������1��DԪ�صĵ���ΪҺ̬�ǽ������ʡ���������Ԫ�ؾ�λ��Ԫ�����ڱ�ǰ�ĸ����ڣ���Ҫ����д���и��⣺
�� AԪ�ص���̬�⻯���õ���ʽ��ʾΪ_______________________________��
DԪ�������ڱ��е�λ��Ϊ_____________________________________��
�� BԪ�ص�����������ˮ�����õ���ʽ��ʾΪ
�� C��D��Ԫ���γɵĻ���������__________������õ���ʽ��ʾ�����ɹ���Ϊ
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com