

 ��
������ ��⺬����������ͭ�Ľ������ϣ�������������������������ͭʧ���ӷ���������Ӧ��Cu-2e-=Cu2+���õ���ɫ��Һ�������ຬ����������������ˮ�����������ܽ⣺Au+HNO3+4HCl=H[AuCl4]+NO��+2H2O��3Pt+4HNO3+18HCl=3H2[PtCl6]+4NO��+8H2O��3Ag+HNO3+3HCl=3AgCl��+NO��+2H2O�����ɰ�ɫ����AgCl����ҺI��AgCl��ˮ�������İ����γ�������Һ����N2H4��Ӧ�õ�Ag���ʣ�
�ͽ��γɻ�����ΪH[AuC14]��H2[PtCl6]����ҺI��ͨ�������Ķ����������壬����������л�ԭ�ԣ�������Ϊ���ᣬ��������ӱ���ԭΪ������2AuCl4-+3SO2+6H2O=2Au+8Cl-+3SO42-+12H+����ҺII����H2[PtCl6]�������Ȼ�粒��壬�õ���NH4��2PtCl6��435�����յõ�Pt��������HCl���ݴ˷������
��� �⣺��1���Ȼ��Ϊ���ӻ��������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2������I�н������������������ӵ�Դ������������һ�����ô�ͭ����Ϊ��ͭ�ľ������ʴ�Ϊ��������ͭ�ľ�����
��3����ˮ��Ũ�����Ũ������ɵĻ��Һ���ǵ��������1��3������ˮ���Ƶľ������Ϊȡһ���Ũ�����������뵽�����Ũ�����У������ò��������裻
�ʴ�Ϊ��ȡһ���Ũ�����������뵽�����Ũ�����У������ò��������裻
��4������������л�ԭ�ԣ�����������ӱ���ԭΪ������Ϊ��ֹ����������ԭ�����������룬SO2����ͨ�������
�ʴ�Ϊ���������Һ�л�ԭ��������ֹ����������ԭ��
��5������������ˮ��Ӧ�����ᱻ��ԭΪNO���漰�ķ�Ӧ�У�Au+HNO3+4HCl=H[AuCl4]+NO��+2H2O��3Pt+4HNO3+18HCl=3H2[PtCl6]+4NO��+8H2O��3Ag+HNO3+3HCl=3AgCl��+NO��+2H2O��
�ʴ�Ϊ��Au+HNO3+4HCl=H[AuCl4]+NO��+2H2O��3Pt+4HNO3+18HCl=3H2[PtCl6]+4NO��+8H2O��3Ag+HNO3+3HCl=3AgCl��+NO��+2H2O����
��6����A���ӵĵ缫�ͻᱻ�Ȼ������ǣ�����A���ӵĵ缫Ϊ��������ӦΪ��Ag-e-+Cl-=AgCl��������B�����ĵ缫Ϊ��������ӦΪ��2H++2e-=H2�������ҳ��в���0.2g ����ʱ������H2Ϊ0.1mol������������0/2mol��ת�Ƶ���Ϊ0.2mol���ʴӼ׳�ת��0.2mol�����ӽ����ҳأ��׳������Ӳ��뷴Ӧ0.2mol���ʼ׳���Һ����0.2mol HCl������Ϊ7.3g��
�ʴ�Ϊ������7.3g��
���� ���⿼�������ʵķ����ᴿ���漰��������ԭ����Ӧ�ã�������ԭ��Ӧ�������жϣ����ԭ����Ӧ�õ�֪ʶ�㣬���������ԭ���ǽ���Ĺؼ����Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ��������������������Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ������������Ư�ס��������ã�ʹ��Ʒ��ɫ�Եð��������ޣ���ͼ����ע�����м�������Na2SO3���壬����������Ũ���ᣨ�Բ��Ӵ�ֽ��Ϊ���������й�˵����ȷ���ǣ�������
������������Ư�ס��������ã�ʹ��Ʒ��ɫ�Եð��������ޣ���ͼ����ע�����м�������Na2SO3���壬����������Ũ���ᣨ�Բ��Ӵ�ֽ��Ϊ���������й�˵����ȷ���ǣ�������| A�� | ��ɫʯ����ֽ������ɫ | |
| B�� | NaOH��Һ�����ڳ�ȥʵ���ж����SO2 | |
| C�� | ʪ�����KI��ֽδ������˵��SO2�л�ԭ�� | |
| D�� | Ʒ����ֽ��պ��KMnO4��Һ����ֽ����ɫ��֤����SO2��Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ��������������������������ʱ����H��0 | |
| B�� | ��H��0��ʾ���ȷ�Ӧ����H��0��ʾ���ȷ�Ӧ | |
| C�� | ��H�Ĵ�С���Ȼ�ѧ����ʽ�еĸ����ʵĻ�ѧ�������� | |
| D�� | �ڻ�ѧ��Ӧ�У��������ʱ仯��ͬʱһ�����������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CH2=CH2�����У�����4���Ҽ���һ���м� | |
| B�� | �ڹ��ۻ������У�һ�����ڼ��Լ������ܴ��ڷǼ��Լ���һ�����������Ӽ� | |
| C�� | N��O��F�縺�Դ�С��F��O��N����һ�����ܴ�С��F��O��N | |
| D�� | ����ǿ����H2SO4��H2SO3��H2SeO3���ҽ��������Ⱥ�˳��SiO2��MgSiO3��CaSiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ������ | ȼ���� | ������ | ȼ���� |
| ���� | 891.0 | ������ | 2878.0 |
| ���� | 1560.8 | �춡�� | 2869.6 |
| ���� | 2221.5 | 2-������ | 3531.3 |
| A�� | ����ȼ�յ��Ȼ�ѧ����ʽΪ��2C2H6��g��+7O2��g��=4CO2��g��+6H2O��g����H=-1560.8 kJ/mol | |
| B�� | �ȶ��ԣ������飾�춡�� | |
| C�� | �������ȼ���ȴ���3531.3kJ/mol | |
| D�� | ��ͬ������������̼����������Խ��ȼ�շų�������Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
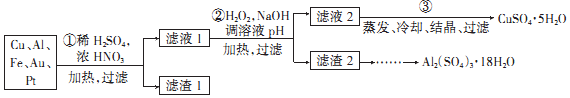
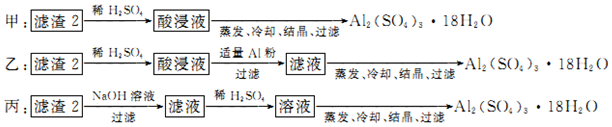
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
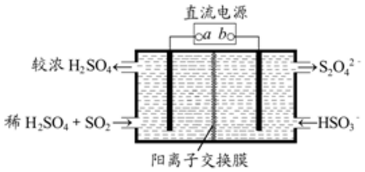 ������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ������NO2������˵����ȷ���ǣ�������
������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ������NO2������˵����ȷ���ǣ�������| A�� | aΪֱ����Դ�ĸ��� | |
| B�� | �� b�缫�����ĵ缫��ӦʽΪ��2HSO3-+2H++2e-�TS2O42-+2H2O | |
| C�� | �� a�����ĵ缫������ԭ��Ӧ�õ�SO42- | |
| D�� | ���ʱ��H+��������ͨ�������ӽ���Ĥ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����Ȼ������гȻ���õ�廨��������ṹ��ʽ��ͼ�����ڸ��л������������ȷ����
����Ȼ������гȻ���õ�廨��������ṹ��ʽ��ͼ�����ڸ��л������������ȷ����| A�� | �ڢܢ� | B�� | �٢ܢ� | C�� | �ڢۢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | �������ʵ | ���� |
| A | С�մ��������Ƹ������ɼ� | Na2CO3��Һ�ʼ��� |
| B | ���������ھ�ˮ | ������ˮ�����ɵ��������������������� |
| C | SiO2����������ά | SiO2�е����� |
| D | SO2��ʹ��ˮ��ɫ | SO2����Ư���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com