
�±�Ϊ���ֶ���������Ԫ�ص������Ϣ��

��ش��������⣺
(1)ZԪ����Ԫ�����ڱ��е�λ���ǵ�________���ڵ�________�壮
(2)Ԫ��T��X��ԭ�Ӹ�����1��1�γɵĻ�����B�����Ļ�ѧ����________(�ѧ������)����֪��ͨ��״���£�39 g��B������H2O��Ӧ�ų�Q kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��________��
(3)��T������������Ӧˮ�������Һ����μ��뵽Y��Z�γɵĻ������ˮ��Һ�У�ֱ������(�ߵμӱ���)���˹����е�������________��
(4)��֪1 mol������A������Na2SO3����Һ�з�����Ӧʱ��ת��2 mol���ӣ�д���÷�Ӧ�����ӷ���ʽ��________��
 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| Ԫ�ش��� | �����Ϣ |
| X | X��ԭ�����������������ڲ������������ |
| Y | �ڵ������ڵ����н��������У�Y�����Ӱ뾶��С |
| Z | Z��Yͬ���ڣ�������������ԭ�Ӱ뾶��С��Ԫ�� |
| T | T�ĵ���������ˮ���ҷ�Ӧ�����ɵ�ǿ���������ֵ�������ȵ����������� |
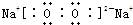
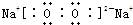
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ����һ�и߶���ѧ��������⻯ѧ�Ծ� ���ͣ������
��10�֣��Ķ��±��в��ֶ���������Ԫ�ص������Ϣ��
��ش�
| Ԫ�ش��� | �����Ϣ |
| X | X��ԭ�����������������ڲ������������ |
| Y | �ڵ������ڵ����ý��������У�Y�����Ӱ뾶��С |
| Z | Z��Yͬ���ڣ�������������ԭ�Ӱ뾶��С��Ԫ�� |
| T | T�ĵ���������ˮ���ҷ�Ӧ�����ɵ�ǿ���������ֵ�������ȵ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����У�ʮһ���У�������һѧ������������ѧ�Ծ� ���ͣ������
10�֣��Ķ��±��в��ֶ���������Ԫ�ص������Ϣ����ش�
|
Ԫ�ش��� |
�����Ϣ |
|
X |
X��ԭ�����������������ڲ������������ |
|
Y |
�ڵ������ڵ����н��������У�Y�����Ӱ뾶��С |
|
Z |
Z��Yͬ���ڣ�������������ԭ�Ӱ뾶��С��Ԫ�� |
|
T |
T�ĵ���������ˮ���ҷ�Ӧ�����ɵ�ǿ���������ֵ�������ȵ����������� |
��1��Ԫ��T��X��ԭ�Ӹ�����1:1�γɵĻ�����B�ĵ���ʽΪ ���û������������Ļ�ѧ����______���ѧ�����ƣ���
��2������T����ˮ��Ӧ�Ļ�ѧ����ʽΪ
��3����Y��Z�γɵĻ������ˮ��Һ����μ��뵽T������������Ӧˮ�������Һ��ֱ���������ߵμӱ�����д���˹����з�����Ӧ�����ӷ���ʽ �� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ�߶���ѧ��������⻯ѧ�Ծ� ���ͣ������
��10�֣��Ķ��±��в��ֶ���������Ԫ�ص������Ϣ��
��ش�
|
Ԫ�ش��� |
�����Ϣ |
|
X |
X��ԭ�����������������ڲ������������ |
|
Y |
�ڵ������ڵ����ý��������У�Y�����Ӱ뾶��С |
|
Z |
Z��Yͬ���ڣ�������������ԭ�Ӱ뾶��С��Ԫ�� |
|
T |
T�ĵ���������ˮ���ҷ�Ӧ�����ɵ�ǿ���������ֵ�������ȵ����������� |
��1��Ԫ��T��X��ԭ�Ӹ�����1:1�γɵĻ�����B�ĵ���ʽΪ ���û������������Ļ�ѧ���� ���ѧ�����ƣ���
��2������T����ˮ��Ӧ�Ļ�ѧ����ʽΪ
��3����Y��Z�γɵĻ������ˮ��Һ����μ��뵽T������������Ӧˮ�������Һ��ֱ���������ߵμӱ�����д���˹����з�����Ӧ�����ӷ���ʽ
�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com