
ŅŃÖŖ£ŗN2(g)£«3H2(g)  2NH3(g)£»”÷H£½£Q kJ”¤mol£1£ØQ£¾0£©”£ĻÖÓŠ¼×ŅŅĮ½øöĻąĶ¬µÄĆܱÕČŻĘ÷£¬Ļņ¼×ČŻĘ÷ÖŠ³äČė1mol N2(g)ŗĶ3mol H2(g)£¬ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±·Å³öµÄČČĮæĪŖQ1 kJ£»ŌŚĻąĶ¬Ģõ¼žĻĀĻņŅŅČŻĘ÷ÖŠ³äČė2mol NH3(g)£¬“ļµ½Ę½ŗāŹ±ĪüŹÕµÄČČĮæĪŖQ2 kJ”£ŅŃÖŖQ2£½3Q1£¬ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ :
2NH3(g)£»”÷H£½£Q kJ”¤mol£1£ØQ£¾0£©”£ĻÖÓŠ¼×ŅŅĮ½øöĻąĶ¬µÄĆܱÕČŻĘ÷£¬Ļņ¼×ČŻĘ÷ÖŠ³äČė1mol N2(g)ŗĶ3mol H2(g)£¬ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±·Å³öµÄČČĮæĪŖQ1 kJ£»ŌŚĻąĶ¬Ģõ¼žĻĀĻņŅŅČŻĘ÷ÖŠ³äČė2mol NH3(g)£¬“ļµ½Ę½ŗāŹ±ĪüŹÕµÄČČĮæĪŖQ2 kJ”£ŅŃÖŖQ2£½3Q1£¬ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ :
A£®Ę½ŗāŹ±¼×ČŻĘ÷ÖŠNH3(g)µÄĢå»ż·ÖŹż±ČŅŅČŻĘ÷ÖŠµÄŠ”
B£®Ę½ŗāŹ±¼×ČŻĘ÷ÖŠĘųĢåµÄŃ¹ĒæĪŖæŖŹ¼Ź±Ń¹ĒæµÄ
C£®“ļµ½Ę½ŗāŹ±£¬¼×ČŻĘ÷ÖŠH2µÄ×Ŗ»ÆĀŹĪŖ25£„
D£®Q1£½Q
 ±¾ĶĮ½ĢøØÓ®ŌŚŹī¼Łøߊ§¼ŁĘŚ×Üø“Ļ°ŌĘÄĻæĘ¼¼³ö°ęÉēĻµĮŠ“š°ø
±¾ĶĮ½ĢøØÓ®ŌŚŹī¼Łøߊ§¼ŁĘŚ×Üø“Ļ°ŌĘÄĻæĘ¼¼³ö°ęÉēĻµĮŠ“š°ø Źī¼Ł×÷Ņµ±±¾©ŅÕŹõÓėæĘѧµē×Ó³ö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷Ņµ±±¾©ŅÕŹõÓėæĘѧµē×Ó³ö°ęÉēĻµĮŠ“š°ø µŚČżŃ§ĘŚÓ®ŌŚŹī¼ŁĻµĮŠ“š°ø
µŚČżŃ§ĘŚÓ®ŌŚŹī¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)”°ÉńÖŪ”±ĮłŗÅĘš·ÉŹ±£¬æÉŅŌ擵½Ņ»¼¶»š¼żµÄÖŠ²æĆ°³öŗģ×ŲÉ«µÄĘųĢ壬ÕāŹĒĪŖĮĖ±£Ö¤ÖüĻä°²Č«Óɱ£ĻÕ»īĆÅ×Ō¶ÆæŖĘōĖłÅųöµÄ²æ·ÖøßŃ¹Ńõ»Æ¼Į±ä»Æ¶ųĄ“µÄ£¬ŅŻ³öµÄŗģ×ŲÉ«ĘųĢåŹĒ_________£¬²śÉśøĆĘųĢåµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ__________________”£
(2)µ±ÓŠ1 molĘ«¶ž¼×ėĀČ¼ÉÕŹ±£¬×ŖŅʵē×ÓµÄĪļÖŹµÄĮæĪŖ_________”£
(3)ÓŠµÄŌĖŌŲ»š¼żÓĆėĀ(N2H4)×÷Č¼ĮĻŗĶ¶žŃõ»ÆµŖ·“Ó¦£¬ŅŃÖŖ£ŗ
N2(g)+2O2(g)====2NO2(g);¦¤H=+67.7 kJ”¤mol-1
N2H4(g)+O2(g)====N2(g)+2H2O(g);¦¤H=-534 kJ”¤mol-1
ĻĀĮŠ¹ŲÓŚėĀŗĶNO2·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£¬ÕżČ·µÄŹĒ( )
A.2N2H4(g)+2NO2(g)====3N2(g)+4H2O(l);¦¤H=-1 135.7 kJ”¤mol-1
B.2N2H4(g)+2NO2(g)====3N2(g)+4H2O(g);¦¤H=-1 000.3 kJ”¤mol-1
C.N2H4(g)+NO2(g)====3/2N2(g)+2H2O(l):¦¤H=-1 135.7 kJ”¤mol-1
D.2N2H4(g)+2NO2(g)====3N2(g)+4H2O(g);¦¤H=-1 135.7 kJ”¤mol-1
Õāøö·“Ó¦ÓĆÓŚ»š¼żĶĘ½ųĘ÷£¬³żŹĶ·Å“óĮæµÄČČŗĶæģĖŁ²śÉś“óĮæĘųĢåĶā£¬»¹ÓŠŅ»øöŗÜ“óµÄÓŵćŹĒ_____________________________________________________________________”£
(4)Ķس£ĒéæöĻĀĖÄŃõ»Æ¶žµŖĘųĢåŗĶ¶žŃõ»ÆµŖĘųĢåæÉĻą»„×Ŗ»Æ£ŗN2O4(g)(ĪŽÉ«)![]() 2NO2(g)(ŗģ×ŲÉ«)£»¦¤H£¾0”£ŅŃÖŖ¶žŃõ»ÆµŖÓŠ½ĻĒæµÄŃõ»ÆŠŌ£¬ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ( )
2NO2(g)(ŗģ×ŲÉ«)£»¦¤H£¾0”£ŅŃÖŖ¶žŃõ»ÆµŖÓŠ½ĻĒæµÄŃõ»ÆŠŌ£¬ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ( )
A.N2O4ŗĶNO2»„ĪŖĶ¬ĖŲŅģŠĪĢå
B.ĘųĢ¬Ź±£¬1 mol N2O4æÉĶźČ«·Ö½ā³É2 mol NO2
C.¼ų±šNO2(g)ŗĶäåÕōĘųæÉŃ”ÓƵķ·Ū”Ŗµā»Æ¼ŲČÜŅŗ
D.N2O4ŗĶNO2¶¼ŹĒĻõĖįµÄĖįōū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)£»¦¤H=-905 kJ”¤mol-1
4NO(g)+6H2O(g)£»¦¤H=-905 kJ”¤mol-1
2H2(g)+O2(g)![]() 2H2O£»¦¤H=-483.6 kJ”¤mol-1
2H2O£»¦¤H=-483.6 kJ”¤mol-1
ŌņN2(g)+3H2(g)![]() 2NH3(g)µÄ¦¤H=________”£
2NH3(g)µÄ¦¤H=________”£
(2)¹¤ŅµÉĻŌŚŅ»¶ØĪĀ¶ČĻĀ£¬½«Ņ»¶ØĮæµÄN2ŗĶH2ĶØČėµ½Ģå»żĪŖ
¢ŁŌö“óŃ¹Ēæ¢ŚŌö“ó·“Ó¦ĪļµÄÅØ¶Č¢ŪŹ¹ÓĆ“ß»Æ¼Į¢Ü½µµĶĪĀ¶Č
(3)µ±»Æѧ·“Ó¦N2(g)+2H2(g) ![]() 2NH3(g)“ļµ½Ę½ŗāŗóøıäijŠ©Ģõ¼ž(²»øıäN2”¢H2ŗĶNH3µÄÓĆĮæ)£¬·“Ó¦ĖŁĀŹÓė·“Ó¦Ź±¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠ±ķŹ¾Ę½ŗā»ģŗĻĪļÖŠNH3µÄŗ¬Įæ×īøßµÄŅ»¶ĪŹ±¼äŹĒ___________________”£µ±ĪĀ¶ČĪŖT ”ꏱ£¬½«
2NH3(g)“ļµ½Ę½ŗāŗóøıäijŠ©Ģõ¼ž(²»øıäN2”¢H2ŗĶNH3µÄÓĆĮæ)£¬·“Ó¦ĖŁĀŹÓė·“Ó¦Ź±¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠ±ķŹ¾Ę½ŗā»ģŗĻĪļÖŠNH3µÄŗ¬Įæ×īøßµÄŅ»¶ĪŹ±¼äŹĒ___________________”£µ±ĪĀ¶ČĪŖT ”ꏱ£¬½«

(4)ŗĻ³É°±µÄŌĮĻĒāĘųŹĒŅ»ÖÖŠĀŠĶµÄĀĢÉ«ÄÜŌ“£¬¾ßÓŠ¹ćĄ«µÄ·¢Õ¹Ē°¾°”£ĻÖÓĆĒāŃõČ¼ĮĻµē³Ų½ųŠŠĻĀĶ¼ĖłŹ¾ŹµŃé£ŗ

¢ŁĒėŠ“³öĒāŃõČ¼ĮĻµē³ŲÖŠµÄµē¼«·“Ó¦Ź½£ŗ
øŗ¼«£ŗ____________________________________________________________£»
Õż¼«£ŗ____________________________________________________________”£
¢ŚÉĻĶ¼×°ÖĆÖŠ£¬Ä³Ņ»Ķµē¼«µÄÖŹĮæ¼õĒįĮĖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(16·Ö) ÄÜŌ“Ī£»śŹĒµ±Ē°Č«ĒņĪŹĢā£¬æŖŌ“½ŚĮ÷ŹĒÓ¦¶ŌÄÜŌ“Ī£»śµÄÖŲŅŖ¾Ł“ė”£
¢ÅĻĀĮŠ×ö·ØÓŠÖśÓŚÄÜŌ“”°æŖŌ“½ŚĮ÷”±µÄŹĒ ”ų £ØĢī×ÖÄø£©”£
a£®“óĮ¦·¢Õ¹Å©“åÕÓĘų£¬½«·ĻĘśµÄ½ÕøŃ×Ŗ»ÆĪŖĒå½ąøߊ§µÄÄÜŌ“
b£®“óĮ¦æŖ²ÉĆŗ”¢ŹÆÓĶŗĶĢģČ»ĘųŅŌĀś×ćČĖĆĒČÕŅęŌö³¤µÄÄÜŌ“ŠčĒó
c£®æŖ·¢Ģ«ŃōÄÜ”¢Ė®ÄÜ”¢·ēÄÜ”¢µŲČȵȊĀÄÜŌ“”¢¼õÉŁŹ¹ÓĆĆŗ”¢ŹÆÓĶµČ»ÆŹÆČ¼ĮĻ
d£®¼õɣ׏Ō“ĻūŗÄ£¬Ōö¼Ó׏Ō“µÄÖŲø“Ź¹ÓĆ”¢×ŹŌ“µÄŃ»·ŌŁÉś
(2)½šøÕŹÆŗĶŹÆÄ«¾łĪŖĢ¼µÄĶ¬ĖŲŅģŠĪĢ壬ĖüĆĒČ¼ÉÕŃõĘų²»×揱ɜ³ÉŅ»Ńõ»ÆĢ¼£¬³ä·ÖČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼£¬·“Ó¦ÖŠ·Å³öµÄČČĮæČēÓŅĶ¼ĖłŹ¾”£
£Øa£©ŌŚĶس£×“æöĻĀ£¬½šøÕŹÆŗĶŹÆÄ«ÖŠ____”ų___£ØĢī”°½šøÕŹÆ”±»ņ”°ŹÆÄ«”±£©øüĪČ¶Ø£¬ŹÆÄ«µÄČ¼ÉÕČČĪŖ____”ų___ kJ”¤mol£1”£
£Øb£©12 gŹÆÄ«ŌŚŅ»¶ØĮææÕĘųÖŠČ¼ÉÕ£¬Éś³ÉĘųĢå36g£¬øĆ¹ż³Ģ·Å³öµÄČČĮæ ”ų kJ”£
(3)ŅŃÖŖ£ŗN2(g)£«O2(g)£½2NO(g)£»¦¤H£½+180.0 kJ”¤mol£1”£
×ŪŗĻÉĻŹöÓŠ¹ŲŠÅĻ¢£¬ĒėŠ“³öCO³żNOµÄČČ»Æѧ·½³ĢŹ½ ”ų ”£
ĆĄ¹śĖ¹Ģ¹ø£“óѧъ¾æČĖŌ±×ī½ü·¢Ć÷Ņ»ÖÖ”°Ė®”±µē³Ų£¬ÕāÖÖµē³ŲÄÜĄūÓƵĖ®Óėŗ£Ė®Ö®¼äŗ¬ŃĪĮæµÄ²ī±š½ųŠŠ·¢µē”£
£Ø4£©ŃŠ¾æ±ķĆ÷£¬µē³ŲµÄÕż¼«ÓƶžŃõ»ÆĆĢÄÉĆ×°ōĪŖ²ÄĮĻæÉĢįøß·¢µēŠ§ĀŹ£¬ÕāŹĒĄūÓĆÄÉĆײÄĮĻ¾ßÓŠ
”ų ĢŲŠŌ£¬ÄÜÓėÄĘĄė×Ó³ä·Ö½Ó“„”£
£Ø5£©ŗ£Ė®ÖŠµÄ”°Ė®”±µē³Ų×Ü·“Ó¦æɱķŹ¾ĪŖ£ŗ5MnO2£«2Ag£«2NaCl£½Na2Mn5O10£«2AgCl£¬øƵē³Ųøŗ¼«·“Ó¦Ź½ĪŖ ”ų £»µ±Éś³É1 mol Na2Mn5O10×ŖŅĘ ”ų molµē×Ó”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģŌĘÄĻŹ”ĆÉ×Ōøß¼¶ÖŠŃ§øßČż1ŌĀŌĀæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ŅŃÖŖ£ŗN2(g)+O2(g)=2NO(g)£»”÷H= +180.5kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)£»”÷H=£905kJ/mol
2H2(g)+O2(g)=2H2O(g)£»”÷H=£483.6kJ/mol
ŌņN2(g)+3H2(g)=2NH3(g)µÄ”÷H= ”£
£Ø2£©¹¤ŅµŗĻ³É°±µÄ·“Ó¦ĪŖN2(g)+3H2(g)  2NH3(g)”£ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬½«Ņ»¶ØĮæµÄN2ŗĶH2ĶØČė¹Ģ¶ØĢå»żĪŖ1LµÄĆܱÕČŻĘ÷ÖŠ“ļµ½Ę½ŗāŗó£¬øıäĻĀĮŠĢõ¼ž£¬ÄÜŹ¹Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ ”£
2NH3(g)”£ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬½«Ņ»¶ØĮæµÄN2ŗĶH2ĶØČė¹Ģ¶ØĢå»żĪŖ1LµÄĆܱÕČŻĘ÷ÖŠ“ļµ½Ę½ŗāŗó£¬øıäĻĀĮŠĢõ¼ž£¬ÄÜŹ¹Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ ”£
¢ŁŌö“óŃ¹Ēæ ¢ŚĶØČėHe
¢ŪŹ¹ÓĆ“ß»Æ¼Į ¢Ü½µµĶĪĀ¶Č
£Ø3£©¹¤ŅµŗĻ³É°±µÄ·“Ó¦ĪŖN2(g)+3H2(g)  2NH3(g)”£ÉčŌŚČŻ»żĪŖ2.0LµÄĆܱÕČŻĘ÷ÖŠ³äČė0.60molN2(g)ŗĶ1.60molH2(g)£¬·“Ó¦ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±£¬NH3µÄĪļÖŹµÄĮæ·ÖŹż£ØNH3µÄĪļÖŹµÄĮæÓė·“Ó¦ĢåĻµÖŠ×ܵÄĪļÖŹµÄĮæÖ®±Č£©ĪŖ
2NH3(g)”£ÉčŌŚČŻ»żĪŖ2.0LµÄĆܱÕČŻĘ÷ÖŠ³äČė0.60molN2(g)ŗĶ1.60molH2(g)£¬·“Ó¦ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±£¬NH3µÄĪļÖŹµÄĮæ·ÖŹż£ØNH3µÄĪļÖŹµÄĮæÓė·“Ó¦ĢåĻµÖŠ×ܵÄĪļÖŹµÄĮæÖ®±Č£©ĪŖ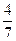 ”£¼ĘĖćøĆĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±N2×Ŗ»ÆĀŹĪŖ £»
”£¼ĘĖćøĆĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±N2×Ŗ»ÆĀŹĪŖ £»
£Ø4£©ŗĻ³É°±µÄŌĮĻĒāĘųŹĒŅ»ÖÖŠĀŠĶµÄĀĢÉ«ÄÜŌ“£¬¾ßÓŠ¹ćĄ«µÄ·¢Õ¹Ē°¾°”£ĻÖÓĆĒāŃõČ¼ĮĻµē³Ų½ųŠŠÓŅĶ¼ĖłŹ¾ŹµŃé£ŗ£ØĘäÖŠc”¢d¾łĪŖĢ¼°ō£¬NaClČÜŅŗµÄĢå»żĪŖ500ml£©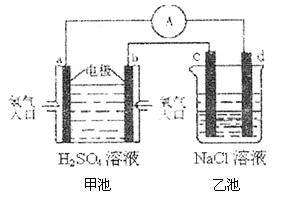
¢Łb¼«ĪŖ ¼«£¬µē¼«·“Ó¦Ź½ £»
c¼«ĪŖ ¼«£¬µē¼«·“Ó¦Ź½ 
¢ŚÓŅĶ¼×°ÖĆÖŠ£¬µ±b¼«ÉĻĻūŗĵÄO2ŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ280mlŹ±£¬ŌņŅŅ³ŲČÜŅŗµÄPHĪŖ £Ø¼ŁÉč·“Ó¦Ē°ŗóČÜŅŗĢå»ż²»±ä£¬ĒŅNaClČÜŅŗ×ćĮ棩
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com