
| A�� | �ɸ���ͬ����Ԫ�صĵ�һ�����ܱ仯���ɣ��Ƴ�Al�ĵ�һ�����ܱ�Mg�� | |
| B�� | �ṹ��������Ƶķ��Ӿ��壬�е�����Է���������������ߣ��Ƴ�NH3�ķе����PH3 | |
| C�� | ������Һ��pH����Һ����ԵĹ�ϵ���Ƴ�pH=6.8����Һһ�������� | |
| D�� | �����ܽ��С�ij��������ܽ�ȸ�С�ij���ת���Ĺ��ɣ��Ƴ���ZnS�����еμ�CuSO4��Һ���Եõ�CuS���� |
���� A��ͬ����Ԫ�أ���һ�����ܵı仯����Ϊ�������ң�������þ�������⣬������þ�ļ۵����Ų���3s2��3p���ȫ�ս��ȶ���������3s23p1������ȫ����ȫ�ա����������һ����������ȶ���
B�������Ӽ�������������Ĵ��ڵ������ʵķе��쳣�ߣ�
C��������ָc��H+����c��OH-������pHʵ�ʷ�ӳ����c��H+�������ܷ�ӳ���ߵ���Դ�С��
D�������ܵ���ʵ��ܽ�ƽ��ĽǶȷ�����
��� �⣺A��ͬ����Ԫ�أ���һ�����ܵı仯����Ϊ�������ң�������þ�������⣬������þ�ļ۵����Ų���3s2��3p���ȫ�ս��ȶ���������3s23p1������ȫ����ȫ�գ����������һ����������ȶ��������ĵ�һ�����ܱ�þС����A����
B�������Ӽ�������������Ĵ��ڵ��·е��쳣�ĸߣ�����NH3�ķе����PH3����B����
C��������ָc��H+����c��OH-������pHʵ�ʷ�ӳ����c��H+����ˮ�����ӻ��������¶�Ӱ�죬��pH=6.8����˵����Һһ�������ԣ���C����
D��ZnS����CuSO4��Һ����ת��Ϊͭ��CuS����˵��CuS�����ܣ���D��ȷ��
��ѡD��
���� ���⿼����Ԫ�������ɡ���Һ����Ե�֪ʶ������ʱҪע��һ���������������Ĺ�ϵ���Լ����������õ������ȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ѹǿ/Mpa ת����/% �¶�/�� | 0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
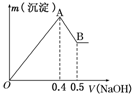 ����AlCl3��MgSO4�Ļ����Һ�������в��ϼ���NaOH��Һ�õ��ij������������NaOH��Һ�������ϵ��ͼ��ʾ����ԭ��Һ��Cl-��SO42-�����ʵ���֮��Ϊ��������
����AlCl3��MgSO4�Ļ����Һ�������в��ϼ���NaOH��Һ�õ��ij������������NaOH��Һ�������ϵ��ͼ��ʾ����ԭ��Һ��Cl-��SO42-�����ʵ���֮��Ϊ��������| A�� | 1��1 | B�� | 2��3 | C�� | 3��2 | D�� | 6��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | Cl2 | Br2 | I2 | H2 | HF | HCl HBr HI |
| ���� | 243 | 193 | 151 | 436 | 568 | 432 366 298 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 mol•L-1��NaHSO3��Һ��0.2 mol•L-1��NaClO��Һ�������ϣ�HSO3-+ClO-=SO42-+Cl-+H+ | |
| B�� | ��Ũ�ȵ�Fe2��SO4��3��Һ��Ba��OH��2��Һ��ϣ�2Fe3++3SO42-+3Ba2++6OH-=2Fe��OH��3��+3BaSO4�� | |
| C�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��2HCO3-+Ca2++2OH-=CaCO3��+CO32-+2H2O | |
| D�� | H218O��Ͷ��������ƣ�2H218O+2Na2O2=4Na++4OH-+18O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H3O+ | B�� | NaOH | C�� | NH4Cl | D�� | H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����¶� | B�� | ʹ�ô��� | ||
| C�� | ����ϡ�����壬������ϵѹǿ | D�� | ����N2��H2����ʼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com