
����Ŀ��A��B��C��D��E�������ʾ�����ͬһ��Ԫ��X������֮��������ת����ϵ��
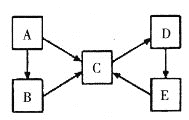
(1)��AΪ���ʣ���B��C�������࣬��A��B��C��Ԫ��X�Ļ��ϼ��������ߣ�C��D��E��Ԫ��X�Ļ��ϼ���ͬ����D����ɫΪ__________��E������Ϊ____________��
(2)��AΪ���ʣ�B��C���������࣬��B��C��ˮ��Һ�к�XԪ�ص��������������֮��Ϊ3��1��D��һ�ְ�ɫ��������Ԫ��X�����ڱ��е�λ����____________��A��C�ķ�Ӧ���������Ļ�ѧʽΪ___________��C��D��Ӧ�����ӷ���ʽΪ__________________________________��
(3)��A~E��Ϊ�����A�ǵ���ɫ���壬C��D��E���������࣬D��E��C���ҹ���ѧ�ҷ����ľ��乤ҵ�Ʊ�C�ķ�������A�ĵ���ʽΪ___________��D��E�Ļ�ѧ����ʽΪ��____________________________________��
(4)��AΪ���ʣ�C��D����Է����������16��B��E������Ӧֻ����һ�ֲ�����������ࡣ��B��C�Ļ�ѧ����ʽΪ____________________________��E��C_________________________________��
���𰸡����ɫ ������ 3����IIIA�� H2O AlO2-+CO2+2H2O=Al(OH)3��+HCO3- ![]() NaCl+CO2+NH3+H2O=NH4Cl+NaHCO3�� 4NH3+5O2
NaCl+CO2+NH3+H2O=NH4Cl+NaHCO3�� 4NH3+5O2![]() 4NO+6H2O 3Cu+8HNO3��ϡ��=3Cu(NO3)2+2NO��+4H2O
4NO+6H2O 3Cu+8HNO3��ϡ��=3Cu(NO3)2+2NO��+4H2O
��������
(1)��AΪ���ʣ���B��C�������࣬��A��B��C��Ԫ��X�Ļ��ϼ��������ߣ�C��D��E��Ԫ��X�Ļ��ϼ���ͬ���ݴ���Ϣ��֪XΪ���Ԫ��������AΪ����BΪ�Ȼ�������CΪ�Ȼ����� DΪ����������EΪ��������������D����ɫΪ���ɫ��E������Ϊ��������
����������������������ɫ����������
(2)��AΪ���ʣ�B��C���������࣬��B��C��ˮ��Һ�к�XԪ�ص��������������֮��Ϊ3��1��D��һ�ְ�ɫ�����������Ϸ�����֪XΪ��Ԫ������AΪ����BΪ������CΪƫ�������� DΪ����������EΪ����������ԭ�ӵĺ˵����Ϊ13�������ڱ��е�λ����3����IIIA�壻����ǿ��ˮ��Һ��Ӧ����ƫ�����κ�������������ԭ����H2O����������ƫ��������Һ��ͨ�������Ķ�����̼���������������������ӷ���ʽΪ��AlO2-+CO2+2H2O=Al(OH)3��+HCO3-������������������ǣ�3����IIIA�壻H2O��AlO2-+CO2+2H2O=Al(OH)3��+HCO3-��
(3)��A~E��Ϊ�����A�ǵ���ɫ���壬Ϊ����������C��D��E���������࣬D��E��C���ҹ���ѧ�ҷ����ľ��乤ҵ�Ʊ�C�ķ������÷���Ϊ�����Ƽ������CΪ̼���ƣ����A ��Na2O2�� B :NaOH�� C :Na2CO3��D : NaCl��E :NaHCO3��Na2O2�������ӻ��������ʽΪ��![]() ���Ȼ�����Һ��ͨ�백����������̼��Ӧ����̼�����ƺ��Ȼ�泥���ѧ����ʽΪ��NaCl+CO2+NH3+H2O=NH4Cl+NaHCO3�����������������������
���Ȼ�����Һ��ͨ�백����������̼��Ӧ����̼�����ƺ��Ȼ�泥���ѧ����ʽΪ��NaCl+CO2+NH3+H2O=NH4Cl+NaHCO3�����������������������![]() ��NaCl+CO2+NH3+H2O=NH4Cl+NaHCO3����
��NaCl+CO2+NH3+H2O=NH4Cl+NaHCO3����
(4)��AΪ���ʣ�C��D����Է����������16��B��E������Ӧֻ����һ�ֲ�����������࣬��������Ϣ��֪����A��N2��B��NH3��C��NO��D��NO2��E��HNO3��������������������һ����������ѧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O��ͭ��ϡ���ᷴӦ��������ͭ��һ����������ѧ����ʽΪ��3Cu+8HNO3��ϡ��=3Cu(NO3)2+2NO��+4H2O���������������������4NH3+5O2
4NO+6H2O��ͭ��ϡ���ᷴӦ��������ͭ��һ����������ѧ����ʽΪ��3Cu+8HNO3��ϡ��=3Cu(NO3)2+2NO��+4H2O���������������������4NH3+5O2![]() 4NO+6H2O��3Cu+8HNO3��ϡ��=3Cu(NO3)2+2NO��+4H2O��
4NO+6H2O��3Cu+8HNO3��ϡ��=3Cu(NO3)2+2NO��+4H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��өʯ(Fluorite)���ֳƷ�ʯ����һ�ֿ������Ҫ�ɷ��Ƿ�����(CaF2)��CaF2����
A.����B.��C.��D.��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ܿ�����ָʾ���ʹ������Ʊ�����ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��A12O3��MnO��MgO��CaO��SiO2�ȣ���ȡCoC2O4��2H2O�����������£�

��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�����������£�ClO3-��������Co2+��ClO3-ת��ΪCl-��
�۲���������������������ʽ����ʱ��Һ��pH������
������ | Fe(OH)3 | Al(OH)3 | Co(OH)2 | Fe(OH)2 | Mn(OH)2 |
��ȫ������pH | 3.7 | 5.2 | 9.2 | 9.6 | 9.8 |
��1�����������м���Na2SO3����ҪĿ����________��
��2�������Һ�м���NaClO3�����ӷ�Ӧ����ʽ��_________��
��3����֪��������NH3��H2O![]() NH4+��OH- Kb��1.8��10-5
NH4+��OH- Kb��1.8��10-5
H2C2O4![]() H+��HC2O4- Ka1��5.4��10-2
H+��HC2O4- Ka1��5.4��10-2
HC2O4-![]() H��C2O42- Ka2��5.4��10-5
H��C2O42- Ka2��5.4��10-5
�������������(NH4)2C2O4��Һ��pH______7�������������=������
��4������(NH4)2C2O4 ��Һ���������壬�ٹ��ˡ�ϴ�ӣ�ϴ��ʱ��ѡ�õ��Լ��У�________��
A������ˮ B������ˮ C�����͵�(NH4)2C2O4��Һ D��ϡ����
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ����ͼ1����ȡ����������________����ʹ�õ�����pH��Χ��________��
A��2.0��2.5 B��3.0��3.5 C��4.0��4.5
��6��CoC2O4��2H2O�ȷֽ������仯������ͼ2��ʾ������600����ǰ�Ǹ����������ȣ�600 ���Ժ����ڿ����м��ȡ�A��B��C��Ϊ�����C����ʾ����Ļ�ѧʽ��________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� A��B��C��D��E��M��N���ֶ���������Ԫ�أ����ǵĺ˵������������A����D��E�γ�10���ӷ��ӣ�����B���������������ڴ�����������Cԭ�������������Ǵ�����������2����M��L�������ΪK���M�������֮�ͣ�D��Mͬ���塣�ش��������⣺
��1��Ԫ��B�ķ��ź����Ʒֱ���____��______�������ڱ��е�λ����_________________��
��2��Ԫ��C��ԭ�ӽṹʾ��ͼΪ______________________________��
��3��Ԫ��C��M���γ�CM2��C��N���γ�CN4�������ֻ�����������ܼ��������ʽ�ֱ�Ϊ��________________��____________________��
��4��Ԫ��A��D��E�γ�10���ӷ��ӵĽṹʽ�ֱ�Ϊ��_______________�� _________________��
��5��Ԫ��D��M��ȣ��ǽ����Խ�ǿ����_____________(��Ԫ�ط��ű�ʾ)��
��6��Ԫ��D��M���⻯��ķе�ߵ�˳��Ϊ��______________________(�û�ѧʽ��ʾ)��
��7����һ�������£�A��D�ĵ��ʺ�M������������Ӧˮ�������Һ�ɹ���ԭ��أ��õ���ڷŵ�����У��������Һ�����Խ�_____________���������С�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ������������Һ�д���������������� �� ��
A.���д���Al3+����Һ��Na+��NH4+��SO42-��Cl-
B.������Һ��Na+��Ca2+��SO42-��CO32-
C.���д���Fe3+����Һ��Na+��Mg2+��NO3-��SCN-
D.���д���NO3-����Һ��H+��Fe2+��SO42-��Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�������ȷ����( )

A. ����ͼ��֪�ϳɼ״����Ȼ�ѧ����ʽΪCO(g)+2H2(g)=CH3OH(g) ��H1=(b-a)kJ��mol-1
B. ͼ�ұ�ʾ2mol H2(g)�����е�������2mol��̬ˮ�����е�������483.6kJ
C. 1mol NaOH�ֱ��1mol CH3COOH��1mol HNO3��Ӧ�����߱�ǰ����HС
D. ����ȼ��ʱ��ȫ���Ļ�ѧ��ת��Ϊ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��������ӵ�ص��ܷ�ӦʽΪ2Li+FeS=Fe+Li2S��LiPF6��SO(CH3)2Ϊ����ʣ��øõ��Ϊ��Դ��⺬�����Է�ˮ���õ�����Ni��ʵ��װ����ͼ��ʾ������˵������ȷ����

A. �缫YΪLi
B. �������У�b��NaCl��Һ�����ʵ���Ũ�Ƚ����ϼ�С
C. X����ӦʽΪFeS+2Li++2e-=Fe+Li2S
D. ����ͼ��������Ĥȥ������a��b���Һϲ������ⷴӦ�ܷ���ʽ�����ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����( )
A. Mg�Ľ����Ա�Alǿ B. H�������Ӱ뾶����Li��
C. HCl�����ȶ��Ա�HFǿ D. HClO4�����Ա�H3PO4ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����()
A. Ħ����һ������������
B. 1mol H2O��������ԭ�ӵ�����Ϊ16g
C. 10L������8L��������H2�����ʵ�����
D. ij���ʺ���6.02��1023������,������ʵ����Ϊ22.4L
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com