
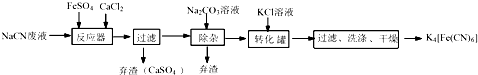
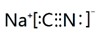 ��
������ ��1��ʵ��Ŀ���Ǻϳɻ�Ѫ�Σ������̿�֪��NaCN��Һ�м��������������Ȼ��ƣ�����6NaCN+FeSO4+CaCl2=Na4[Fe��CN��6+CaSO4��+2NaCl��Ȼ�����̼������Һ�ɳ�ȥ������Ca2+�������˺�����Һ�м���KCl��ת������K4[Fe��CN��6���Դ˽����⣻
��2������${K}_{h}=\frac{c��HCN��c��O{H}^{-}��}{c��C{N}^{-}��}$�����ˮ��ƽ�ⳣ�����Ƚ�NaCN��ˮ��ƽ�ⳣ����HCN�ĵ���ƽ�ⳣ�����ж���Һ������ԣ�
��3�����������֪��NaCN��Һ��Au�Լ������е�������Ӧ��Na[Au��CN��2]�� ����Ͻ����ƣ���Һ������Ԫ���غ����д��ѧ����ʽ�����ݵ��ӵ�ʧ�غ�ɼ�������ĵ�п�����ɵĽ�����ʵ���֮�ȣ�
��4��ÿĦ��NaCN����ΪN2�ͺ�NaHCO3����Ҫת��5mol���ӣ�1mol O3�õ�2mole-�����ݵ��ӵ�ʧ�غ�ɼ������״���µ�O3 �������
��� �⣺��1��ʵ��Ŀ���Ǻϳɻ�Ѫ�Σ������̿�֪��NaCN��Һ�м��������������Ȼ��ƣ�����6NaCN+FeSO4+CaCl2=Na4[Fe��CN��6+CaSO4��+2NaCl��Ȼ�����̼������Һ�ɳ�ȥ������Ca2+�������˺�����Һ�м���KCl��ת������K4[Fe��CN��6��
��NaCNΪ���ӻ��������ʽΪ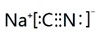 ��
��
�ʴ�Ϊ��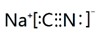 ��
��
��NaCN��Һ�м��������������Ȼ��ƣ�����6NaCN+FeSO4+CaCl2=Na4[Fe��CN��6+CaSO4��+2NaCl��
�ʴ�Ϊ��6NaCN+FeSO4+CaCl2=Na4[Fe��CN��6+CaSO4��+2NaCl��
�۷�Ӧ���м����Ȼ��ƣ�����̼������Һ�ɳ�ȥ������Ca2+��
�ʴ�Ϊ����ȥ���е�Ca2+��
��2������${K}_{h}=\frac{c��HCN��c��O{H}^{-}��}{c��C{N}^{-}��}$��֪��${K_h}=\frac{{c��HCN��•c��O{H^-}��}}{{c��C{N^-}��}}=\frac{{c��HCN��•c��O{H^-}��•c��{H^+}��}}{{c��C{N^-}��•c��{H^+}��}}=\frac{K_W}{K_a}=\frac{{1��{{10}^{-14}}}}{{6.2��{{10}^{-10}}}}$=1.6��10-5��6.2��10-10����ˮ��ƽ�ⳣ�����ڵ���ƽ�ⳣ����������Һ�ʼ��ԣ�
�ʴ�Ϊ�����ԣ�${K_h}=\frac{{c��HCN��•c��O{H^-}��}}{{c��C{N^-}��}}=\frac{{c��HCN��•c��O{H^-}��•c��{H^+}��}}{{c��C{N^-}��•c��{H^+}��}}=\frac{K_W}{K_a}=\frac{{1��{{10}^{-14}}}}{{6.2��{{10}^{-10}}}}$=1.6��10-5��6.2��10-10��
��3�����������֪��NaCN��Һ��Au�Լ������е�������Ӧ��Na[Au��CN��2]�� ����Ͻ����ƣ���Һ����Ӧ�Ļ�ѧ����ʽΪ4Au+8NaCN+2H2 O+O2=4Na[Au��CN��2]+4NaOH����ԭ1mol��Ҫת��1mol���ӣ���ÿmolп��ת��2mol���ӣ��������ĵ�п�����ɵĽ�����ʵ���֮��Ϊ1��2��
�ʴ�Ϊ��4Au+8NaCN+2H2 O+O2=4Na[Au��CN��2]+4NaOH��1��2��
��4��ÿĦ��NaCN����ΪN2�ͺ�NaHCO3����Ҫת��5mol���ӣ�1mol O3�õ�2mole-�����ݵ��ӵ�ʧ�غ��֪����Ҫ������NaCN0.001mol•L-1103L��������Ҫ��״���µ�O3 �����Ϊ$\frac{5}{2}$��0.001mol•L-1��103L��22.4L•mol-1=56L��
�ʴ�Ϊ��56��
���� ���⿼�����ʵ��Ʊ�ʵ�鷽������ƣ�������ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬Ϊ�߿��������ͣ�ע���������ͼ����ʵ���ԭ���Ͳ���������ע�����õ����غ���м��㣬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܳ��ⶼ���� | B�� | ���ڢ��ⶼ���� | C�� | ֻ�Т٢ݢ��� | D�� | ȫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����£�1molC6H1206����ԭ����Ϊ6NA | |
| B�� | 0.1moICl2������ˮ��ֻ�ϣ�ת�Ƶ�����Ϊ0.1NA | |
| C�� | 25�棬pH=13��Ba��OH��2��Һ����OH-��ĿΪ0.2NA | |
| D�� | 22.4L N2��NH3������庬���õ��Ӷ���ĿΪ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1NaHCO3��Һ�У�c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-�� | |
| B�� | ������������ʵ���Ũ�ȵ�NaX������HX��Ϻ�����Ե���Һ�У�c��X-����c��Na+����c��HX����c��H+����c��OH-�� | |
| C�� | ��0.2 mol•L-1 NaA��Һ��0.1 mol•L-1��������������ü�����Һ�У�c��Na+��+c��H+��=c��A-��+c��Cl-��+c��OH-�� | |
| D�� | 1.5 L 0.1 mol•L-1 NaOH��Һ�л���ͨ��CO2����Һ����4.4 gʱ����Һ�У�c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
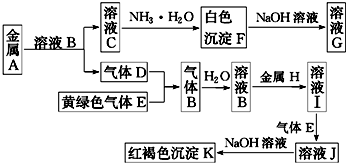
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
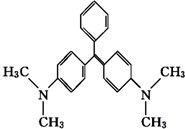 ��ȸʯ���ǻ�����Ʒ�����нϸ߶��ԣ��߲����������°����»�����ṹ��ʽ��ͼ��ʾ�����й��ڿ�ȸʯ�̵�˵����ȷ���ǣ�������
��ȸʯ���ǻ�����Ʒ�����нϸ߶��ԣ��߲����������°����»�����ṹ��ʽ��ͼ��ʾ�����й��ڿ�ȸʯ�̵�˵����ȷ���ǣ�������| A�� | ��ȸʯ�̵ķ���ʽΪC23H25N2 | |
| B�� | 1 mol��ȸʯ����һ��������������6 mol H2�����ӳɷ�Ӧ | |
| C�� | ��ȸʯ�����ڷ����廯���� | |
| D�� | ��ȸʯ�̱����ϵ�һ��ȡ������6�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com