
£Ø·Ö£©ĪļÖŹA”«EµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾:
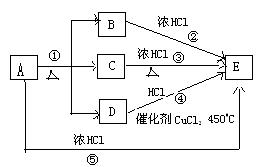
ŅŃÖŖĘųĢåµ„ÖŹDÄÜŹ¹“ų»šŠĒµÄľĢõø“Č¼,·“Ó¦¢ŁŹĒŹµŃéŹŅÖʵ„ÖŹDµÄ³£ÓĆ·½·ØÖ®Ņ»,·“Ó¦¢ŚŹĒŹµŃéŹŅÖĘ»ĘĀĢÉ«ĘųĢåEµÄÖŲŅŖ·“Ó¦,(²æ·ÖÉś³ÉĪļĪ“ĮŠ³ö)”£
ŅĄ¾ŻÉĻŹöŠÅĻ¢,»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AµÄ»ÆѧŹ½
(2) “ÓĀČŌŖĖŲ»ÆŗĻ¼ŪµÄ±ä»Ææ“£¬ŅŌÉĻŠ“³ö·“Ó¦¢Ś¢Ū¢Ü¢ŻĖÄÖÖ·½·ØµÄ¹²Ķ¬µćŹĒ
(3)Š“³ö·“Ó¦¢ÜµÄ»Æѧ·½³ĢŹ½
(4)ĄūÓĆ·“Ó¦¢Ū¢Ü¢ŻµÄ±ä»Æ£¬²»Š“»Æѧ·½³ĢŹ½±Č½ĻA”¢C”¢DµÄŃõ»ÆŠŌÓɓ󵽊”µÄĖ³Šņ
(5)Š“³ö·“Ó¦¢ŚµÄĄė×Ó·½³ĢŹ½ ,Ńõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±Č
£Ø1£©KMnO4
£Ø2£©ĀČŌŖĖŲµÄ»ÆŗĻ¼ŪÓÉ-1¼ŪÉżøßµ½0¼Ū
£Ø3£©4HCl+O2![]() 2Cl2+2H2O
2Cl2+2H2O
£Ø4£©KMnO4>MnO2>O2
(5) MnO2£«4H++2Cl-![]() Mn2++Cl2”ü+2H2O£» 1:2
Mn2++Cl2”ü+2H2O£» 1:2
±¾ĢāŅŌŃõĘų”¢ĀČĘųµÄŹµŃéŹŅÖʱøĪŖĖŲ²Ä£¬×¢ŅāĮ½ÕßÖʱø·½·Ø¾łĪŖŃõ»Æ»¹Ō·“Ó¦£¬¶žÕ߶¼ŅŌøßĆĢĖį¼ŲĪŖ·“Ó¦Īļ£¬ÕāŹĒ½āĢāµÄĶ»ĘĘæŚ”£ŗÜĻŌČ»£¬»ĘĀĢÉ«ĘųĢåµ„ÖŹĪŖĀČĘų£¬µ„ÖŹDĪŖŃõĘų£¬AĪŖøßĆĢĖį¼Ų”£æņĶ¼µÄÖÕµćĪļÖŹĪŖĀČĘų£¬¶¼ŹĒŗĶŃĪĖį·“Ó¦£¬ĖłŅŌ£¬¢Ś¢Ū¢Ü¢ŻĖÄøö·“Ó¦µÄ¹²Ķ¬µćĪŖÓĆ²»Ķ¬µÄŃõ»Æ¼Į¾ł½«ÅØŃĪĖįŃõ»ÆĪŖĀČĘų£¬ĀČŌŖĖŲµÄ»ÆŗĻ¼ŪÓÉ-1¼ŪÉżøßµ½0¼Ū”£øł¾Ż¢Ū¢Ü¢ŻČżøö·“Ó¦ÖŠ·“Ó¦Ģõ¼žµÄ²īŅģ£Ø³£ĪĀ·“Ó¦”¢¼ÓČČ”¢¼ÓČČ“ß»Æ¼Į£©£¬µĆ³öA”¢C”¢DµÄŃõ»ÆŠŌÓɓ󵽊”µÄĖ³ŠņĪŖKMnO4>MnO2>O2”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(12·Ö)A”«Gø÷ĪļÖŹ¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠB”¢DĪŖĘųĢ¬µ„ÖŹ”£
MnO2¢ŁFeµćČ¼
MnO2¢Ś”÷
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪļÖŹCŗĶEµÄĆū³Ę·Ö±šĪŖ________”¢________£»
(2)æÉŃ”ÓĆ²»Ķ¬µÄA½ųŠŠ·“Ó¦¢Ł£¬ČōÄÜŌŚ³£ĪĀĻĀ½ųŠŠ£¬Ęä»Æѧ·½³ĢŹ½___________£»
ČōÖ»ÄÜŌŚ¼ÓČČĒéæöĻĀ½ųŠŠ£¬Ōņ·“Ó¦ĪļAÓ¦ĪŖ________£»
(3)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ_____________________£»
(4)ŠĀÅäÖʵÄFČÜŅŗÓ¦¼ÓČė________ŅŌ·ĄÖ¹Ęä×Ŗ»ÆĪŖG”£¼ģŃéGČÜŅŗÖŠŃōĄė×ӵij£ÓĆŹŌ¼Į
ŹĒ________£¬ŹµŃéĻÖĻóĪŖ_____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(10·Ö)A”«Gø÷ĪļÖŹ¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠB”¢DĪŖĘųĢ¬µ„ÖŹ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪļÖŹCŗĶEµÄĆū³Ę·Ö±šĪŖ________________”¢__________________£»
(2)æÉŃ”ÓĆ²»Ķ¬µÄA½ųŠŠ·“Ó¦¢Ł£¬ČōÄÜŌŚ³£ĪĀĻĀ½ųŠŠ£¬Ęä»Æѧ·½³ĢŹ½ĪŖ_____________£»
ČōÖ»ÄÜŌŚ¼ÓČČĒéæöĻĀ½ųŠŠ£¬Ōņ·“Ó¦ĪļAÓ¦ĪŖ_____________£»
(3)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________£»
(4)ŠĀÅäÖʵÄFČÜŅŗÓ¦¼ÓČė_____________ŅŌ·ĄÖ¹Ęä×Ŗ»ÆĪŖG”£¼ģŃéGČÜŅŗÖŠŃōĄė×ӵij£ÓĆŹŌ¼ĮŹĒ_____________£¬ŹµŃéĻÖĻóĪŖ_____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗ£ÄĻŹ”¼Ī»żÖŠŃ§øßŅ»ÉĻѧʌ½Ģѧ֏Įæ¼ą²āČż»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)A”«Gø÷ĪļÖŹ¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠB”¢DĪŖĘųĢ¬µ„ÖŹ”£
MnO2¢ŁFeµćČ¼
MnO2¢Ś”÷
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪļÖŹCŗĶEµÄĆū³Ę·Ö±šĪŖ________”¢________£»
(2)æÉŃ”ÓĆ²»Ķ¬µÄA½ųŠŠ·“Ó¦¢Ł£¬ČōÄÜŌŚ³£ĪĀĻĀ½ųŠŠ£¬Ęä»Æѧ·½³ĢŹ½___________£»
ČōÖ»ÄÜŌŚ¼ÓČČĒéæöĻĀ½ųŠŠ£¬Ōņ·“Ó¦ĪļAÓ¦ĪŖ________£»
(3)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ_____________________£»
(4)ŠĀÅäÖʵÄFČÜŅŗÓ¦¼ÓČė________ŅŌ·ĄÖ¹Ęä×Ŗ»ÆĪŖG”£¼ģŃéGČÜŅŗÖŠŃōĄė×ӵij£ÓĆŹŌ¼Į
ŹĒ________£¬ŹµŃéĻÖĻóĪŖ_____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äźøßæ¼»ÆѧŹŌĢā·ÖĻī×ØĢāŹ®Ņ» ½šŹōŌŖĖŲ¼°Ęä»ÆŗĻĪļ ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)A”«Gø÷ĪļÖŹ¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠB”¢DĪŖĘųĢ¬µ„ÖŹ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪļÖŹCŗĶEµÄĆū³Ę·Ö±šĪŖ________________”¢__________________£»
(2)æÉŃ”ÓĆ²»Ķ¬µÄA½ųŠŠ·“Ó¦¢Ł£¬ČōÄÜŌŚ³£ĪĀĻĀ½ųŠŠ£¬Ęä»Æѧ·½³ĢŹ½ĪŖ_____________£»
ČōÖ»ÄÜŌŚ¼ÓČČĒéæöĻĀ½ųŠŠ£¬Ōņ·“Ó¦ĪļAÓ¦ĪŖ_____________£»
(3)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________£»
(4)ŠĀÅäÖʵÄFČÜŅŗÓ¦¼ÓČė_____________ŅŌ·ĄÖ¹Ęä×Ŗ»ÆĪŖG”£¼ģŃéGČÜŅŗÖŠŃōĄė×ӵij£ÓĆŹŌ¼ĮŹĒ_____________£¬ŹµŃéĻÖĻóĪŖ______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌ»ÆѧŹŌĢā£Øŗ£ÄĻ¾ķ£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)A”«Gø÷ĪļÖŹ¼äµÄ¹ŲĻµČēĻĀĶ¼£¬ĘäÖŠB”¢DĪŖĘųĢ¬µ„ÖŹ”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪļÖŹCŗĶEµÄĆū³Ę·Ö±šĪŖ________________”¢__________________£»
(2)æÉŃ”ÓĆ²»Ķ¬µÄA½ųŠŠ·“Ó¦¢Ł£¬ČōÄÜŌŚ³£ĪĀĻĀ½ųŠŠ£¬Ęä»Æѧ·½³ĢŹ½ĪŖ_____________£»
ČōÖ»ÄÜŌŚ¼ÓČČĒéæöĻĀ½ųŠŠ£¬Ōņ·“Ó¦ĪļAÓ¦ĪŖ_____________£»
(3)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________£»
(4)ŠĀÅäÖʵÄFČÜŅŗÓ¦¼ÓČė_____________ŅŌ·ĄÖ¹Ęä×Ŗ»ÆĪŖG”£¼ģŃéGČÜŅŗÖŠŃōĄė×ӵij£ÓĆŹŌ¼ĮŹĒ_____________£¬ŹµŃéĻÖĻóĪŖ______________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com