
���� ����һ����1���μ�����������������Һ��HCO3-��CO32-��ȫת��Ϊ�������ؼ��Ǹó������ܹ���̼��������ӷ�Ӧ���ɳ�����
��2�����ݹ��˲����ķ����ж���Ҫʹ�õ��������ƣ�
��3������δ�����־ͳ����������ֵƫ��������������ȵ�������̼�����Ʋ����ij�������̼���Ʋ����ij������ݴ��ж϶Բⶨ�����Ӱ�죻
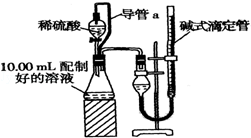
����������1������̼�������ᷴӦ����������̼���壬ƿ��ѹǿ��������װ���е���a��������ƽ��ѹǿ��ʹҺ��˳�����£���������ϡ��������������
��2�������DZ�����������ѹ��ͬ������ע������Ϊ���ٴ���ȴ�����²ſ�ʼ�������ڶ���ǰ����Һ����ƽ��
��3����ʽ�ζ�����ǰ��������Ƕ�����̼��������ݴ˼����������̼�������
����������1��̼���ơ�̼�����ƵĻ��Һ�Լ��ԣ�Ӧ���ü�ʽ�ζ�����ȡ��
��2����̪�ı�ɫ��Χ��8.2��10.0����Ӧ����ʱ��Һ�ɺ�ɫͻ��Ϊ��ɫ���ݴ��жϵζ��յ㣻
��3�����ݷ�ӦH++CO32-=HCO3-�����50 mL��Ʒ��Һ�к��е�̼���Ƶ����ʵ�����Ȼ������250mL��Ʒ��Һ�к��е�̼���Ƶ����ʵ������ٸ���m=nM�����̼���Ƶ����������������������ı���ʽ�������Ʒ��̼���Ƶ�����������
��� �⣺����һ����1��A��CaCl2��Һֻ����̼�����Ӧת��Ϊ̼�����������A����
B��MgSO4��Һ��HCO3-��CO32-������Ӧ����B����
C��NaCl��Һ��HCO3-��CO32-������Ӧ����C����
D��Ba��OH��2��Һ��HCO3-��CO32-��������Ӧ����̼�ᱵ��������D��ȷ��
�ʴ�Ϊ��D��
��2�����˲�������Ҫ�IJ��������У��ձ�������������ͨ©�������Գ��ձ��⣬���в���������ͨ©����©����������˲�������Ҫ�IJ����������ձ��Ͳ����������ͨ©����©������
�ʴ�Ϊ����ͨ©����©������
��3������˲��У�����δ�����־ͳ����������ֵƫ��������������ȵ�������̼�����Ʋ����ij�������̼���Ʋ����ij����������������ֵƫ����̼�����Ƶ�����ƫ����̼���Ƶĺ���ƫС���ʴ�Ϊ��ƫС��
����������1������̼�������ᷴӦ����������̼���壬ƿ��ѹǿ��������װ���е���a��������ƽ��ѹǿ��ʹҺ��˳�����£���������ϡ������������������ȥ����a��ʹ����������ƫ��
�ʴ�Ϊ��ƽ��ѹǿ��ʹҺ��˳�����£���������ϡ������������������ƫ��
��2�������DZ�����������ѹ��ͬ������ע������Ϊ���ٴ���ȴ�����²ſ�ʼ�������ڶ���ǰ����Һ����ƽ����Ҫ��֤���۾�������Һ����ʹ���ƽ��
�ʴ�Ϊ������ȴ�����²ſ�ʼ����������ǰ����Һ����ƽ��
��3����ʽ�ζ�����ǰ��������Ƕ�����̼������������CO2�����Ϊ����V1-V2��mL��
�ʴ�Ϊ����V1-V2����
����������1����Һ�Լ��ԣ����Ӧ���ü�ʽ�ζ�����ȡ��
�ʴ�Ϊ����ʽ�ζ��ܣ�
��2����̪�ı�ɫ��Χ��8.2��10.0�������жϵζ��յ���������ɺ�ɫͻ��Ϊ��ɫ����30s���ָ���
�ʴ�Ϊ���ɺ�ɫͻ��Ϊ��ɫ����30s���ָ���
��3����Ӧ�ﵽ�յ�ʱ�����������ӷ���ʽΪ��H++CO32-=HCO3-��
�������������������֪20.00ml��֪������̼���Ƶ����ʵ���Ϊ��0.2000 mol/L��0.02000L=0.004mol��
��ԭ�������̼���Ƶ����ʵ���Ϊ��0.004mol��$\frac{250ml}{25ml}$=0.04mol������Ϊ��0.04mol��106g/mol=4.24g��
����̼������������Ϊ��$\frac{4.24g}{5.0g}$��100%=84.8%��
�ʴ�Ϊ��84.8��
���� ���⿼��̼���ƺ�̼�����ƻ������̼���ƺ����ⶨ��ʵ�鷽����������ۣ���Ŀ�Ѷ��еȣ���ȷ��ѧʵ���������������̼���ơ�̼�����Ƶ�����Ϊ������ؼ��������ֿ�����ѧ���ķ�����������������ѧʵ�顢��ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
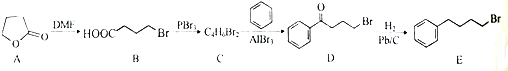
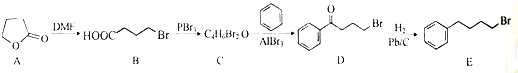
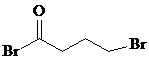 ����C��D�ķ�Ӧ������ȡ����Ӧ��
����C��D�ķ�Ӧ������ȡ����Ӧ��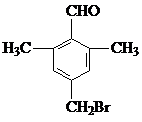 �ȣ�
�ȣ�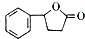 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ������
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
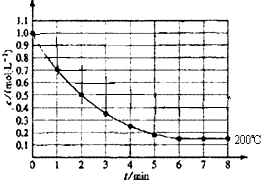 ��һ�ܱ������з���1molX��g��������Ӧ��X��g��?4Y��g��+Z��g������ͼ��ʾ�� 200��ʱ��X��Ũ����ʱ��仯�����ߣ�
��һ�ܱ������з���1molX��g��������Ӧ��X��g��?4Y��g��+Z��g������ͼ��ʾ�� 200��ʱ��X��Ũ����ʱ��仯�����ߣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H5OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O | |
| B�� | CH3OH+HCl��CH3Cl+H2O | |
| C�� | ��NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl | |
| D�� | CH3CH2OH$��_{170��}^{Ũ����}$CH2�TCH2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 0.1 mol•L-1 NaHCO3��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�������Һ�У�c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| B�� | 0.1 mol•L-1Na2CO3��Һ��0.1 mol•L-1NaHCO3��Һ�������ϣ�������Һ�У�c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| C�� | 0.1 mol•L-1CH3COONa��Һ��0.1 mol•L-1CH3COOH��Һ�������Ϻ���Һ�����ԣ�������Һ�У�c��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-�� | |
| D�� | 0.10mol•L-1CH3COONa��Һ��ͨ��HCl�����ԣ�������Һ�У�c��Na+����c��CH3COOH��=c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com