
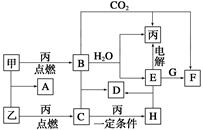
�ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ��
��ش��������⣺
(1)�û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
(2)A�ĵ���ʽΪ________________________________��
(3)���E��ˮ��Һʱ��E��������________________________��
(4)д��B��C����D�Ļ�ѧ����ʽ��_____________________________��
д��E��G����F�����ӷ���ʽ��__________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡТ���и����ϵ�һ��ͳ����ѧ�Ծ��������棩 ���ͣ�ѡ����
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ��( )
A����NaAlO2��Һ����NaHCO3��Һ��HCO3-��AlO2-��H2O==CO32-��Al(OH)3��
B��ʵ�����Ʊ������������壺Fe3����3H2O==Fe(OH)3����3H��
C����FeBr2��Һ��ͨ�����������2Fe2����2Br����2Cl2==2Fe3����Br2��4Cl��
D��Na2O2����ˮ����O2��Na2O2��H2O===2Na����2OH����O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�����и߶���ѧ��ѧҵģ������ѧ�Ծ��������棩 ���ͣ�ѡ����
��ҵ����������е�һ����Ҫ��ӦΪ4NH3��5O2 4NO��6H2O�������йظ÷�Ӧ��˵����ȷ����
4NO��6H2O�������йظ÷�Ӧ��˵����ȷ����
A��O2�ǻ�ԭ�� B��NH3��������
C��O2ʧȥ���� D��NH3����������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�����и߶���ѧ��ѧҵģ������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����˿ڵġ�ǧ���������ɽ���һ���������У�������ǻ벻�£�Ҫ��������˼䡣��������������ǫ��������ʯ�����������ľ���û���漰��ѧ�仯����
A��ǧ���������ɽ B���һ����������
C��������ǻ벻�� D��Ҫ��������˼�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ���������ݸ�����һ12���¿���ѧ���������棩 ���ͣ�ʵ����
�Ը������������Ĺ�ҵ��ҺΪԭ�������������Ĺ�������(���ֲ�����������)��
��.�ӷ�Һ���ᴿ���ᾧ��FeSO47H2O��
��.��FeSO47H2O���Ƴ���Һ��
��.FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3����Һ��
��.����Һ���ˣ���90����ˮϴ�ӳ����� �����õ�FeCO3���塣
�����õ�FeCO3���塣
��.����FeCO3���õ�Fe2O3���塣
��֪��NH4HCO3����ˮ�зֽ⡣
(1)���У�����������м��ȥ��Һ�е�Fe3+���÷�Ӧ�����ӷ���ʽ��_________________��
(2)���У����һ�������ᡣ���û�ѧƽ��ԭ���������������_____________��
(3)���У�����FeCO3�����ӷ���ʽ��_____________����FeCO3��Һ��ʱ�䱩¶�ڿ����У����в��ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ��_____________��
(4)���У�ͨ������SO42-���жϳ����Ƿ�ϴ�Ӹɾ�������SO42-�IJ�����_____________��
(5)��֪����FeCO3�Ļ�ѧ����ʽ��4FeCO3+O2 2Fe2O3+4CO2��������464.0kg��FeCO3,�õ�316.8kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������_________kg��(Ħ������/gmol-1:FeCO3116Fe2O3160FeO72)
2Fe2O3+4CO2��������464.0kg��FeCO3,�õ�316.8kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������_________kg��(Ħ������/gmol-1:FeCO3116Fe2O3160FeO72)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ���������ݸ�����һ12���¿���ѧ���������棩 ���ͣ�ѡ����
��Al2(SO4)3��K2SO4�������Ļ����Һ��,���c(SO42-)����0.2mol/L,������������0.2mol/L��KOH��Һʱ,���ɵij���ǡ���ܽ�,��ԭ�����Һ��K+�����ʵ���Ũ��Ϊ
A��0.2mol/L B��0.25mol/L C��0.45mol/L D��0.225mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ���������ݸ�����һ12���¿���ѧ���������棩 ���ͣ�ѡ����
һ������������Һ�д����������������
�ٺ��д���Al3+����Һ�У�Na+��NH4+��SO42-��Cl-
�ڼ���Al�ܷų�H2����Һ�У�Cl-��CO32-��SO42-��NH4+
�ۺ��д���Fe3������Һ�У�Na����Mg2����NO3-��SCN��
���ں��д���AlO2-����Һ�У�NH4+��Na����Cl����H��
����ˮ�������c(H��)��1��10��14molL��1����Һ�У�Ca2����K����Cl����HCO3
A���� B���٢� C���٢ۢ� D���٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�������һ12���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ȤС������ͼװ��̽�����Ĵ���������������������������
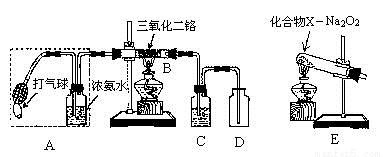
��1�����������Ļ�ѧ����ʽΪ _________________________________________��
��2�����Ȳ�����Bһ��ʱ���ѹA�д��������������۲쵽B�����ʳʺ���״̬��ֹͣ���Ⱥ����ܱ��ֺ��ȣ��÷�Ӧ��_______��Ӧ������ȡ����ȡ�����
��3��Ϊ��֤��װ��D�й۲쵽����ɫ���壬װ��CӦװ��_____________����ȡ��C����D�н��۲쵽�������̣�ԭ����_________________________��
��4��Ϊʵ�ְ���������Ҳ����װ��E�滻װ��_____________(��װ�ô���)��������XΪ___________(�ѧʽֻдһ��)��Na 2 O 2 ��������_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶�����ĩ��ѧ���������棩 ���ͣ�ѡ����
�������������ͬ��������Һ����pH=3��CH3COOH��Һ ��pH=3������ ��pH=11�İ�ˮ��pH=11��NaOH��Һ������˵����ȷ����
A������������Һϡ��100������ ҺpH��С˳��>��>��>��
ҺpH��С˳��>��>��>��
B���ۺֱܷ͢��õ�Ũ�ȵ�������Һ�кͣ�����������Һ���������=��
C������ڷֱ�������þ�۷�Ӧ������H2��������<��
D���ں͢ۻ�ϣ� ���û����Һ��pH����7
���û����Һ��pH����7
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com