
һ��ѧ�о���ѧϰС���ijNa2CO3��NaHCO3�����Һ����ɽ���̽����ȡ20.0 mL�û����Һ���ϼ���1.00 mol��L��1��ϡ���ᣬ�������������Ͳ�����������±���
| ��Ӧ�� | �� | �� | �� |
| �������x/mL | 0<x��10.0 | 10.0<x��40.0 | x>40.0 |
| ���� | ������ | �������� | ������ |
������Һ��c(HCO )Ϊ (����)��
)Ϊ (����)��
A��1.00 mol��L��1�� B��0.50 mol��L��1
C��1.50 mol��L��1�� D��2.00 mol��L��1
��������Ӧ��CO ��H��===HCO
��H��===HCO ����Ӧ��HCO
����Ӧ��HCO ��H��===
��H��===
CO2����H2O����20.0 mL�û����Һ��Na2CO3��NaHCO3�����ʵ����ֱ�Ϊ��x mol��y mol������x mol��1.00 mol��L��1��10.0��10��3 L��0.01 mol��x mol��y mol��1.00 mol��L��1��(40.0��10.0)��10��3 L��0.03 mol�����x��0.01��y��0.02����c(HCO )��1.00 mol��L��1��
)��1.00 mol��L��1��
�𰸡�A



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����в�������Ӧ��һ�������� (����)��
�ٹ���NaOH��Һ���ڹ������ᡡ�۹�������ᡡ�ܶ���������̼���ơ��ݶ���������NaOH��Һ������������Ũ����
A���٢ڢܡ� B���ۢܢ�
C���ڢݢޡ� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������KSCN(SCN���ǡ���±���ӡ�)��Һ���뵽�����ữ����������Һ�У���Һ��ɺ�ɫ�����ú�ɫ��Һ��Ϊ���ݣ���һ���м�������KMnO4��Һ����ɫ��ȥ��������һ����ͨ��SO2����ɫҲ��ȥ�������Ʋ�϶�����ȷ���� (����)��
A�����к�ɫ��ȥ��ԭ����KMnO4��SCN������
B�����к�ɫ��ȥ��ԭ����SO2��Fe3����ԭ��Fe2��
C�����к�ɫ��ȥ��ԭ����SO2��SCN����ԭ
D��SCN�����ʵ������¿�ʧȥ���ӱ�����Ϊ(SCN)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮAlCl3���������������л��ϳɵĴ����ȡ���ҵ����������(Al2O3��Fe2O3)Ϊԭ���Ʊ���ˮAlCl3�Ĺ����������¡�
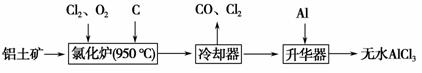
(1)�Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽΪ_____________________ _________________________________________________________��
(2)��Na2SO3��Һ�ɳ�ȥ��ȴ���ų���β���е�Cl2���˷�Ӧ�����ӷ���ʽΪ_______________________________________________________________��
(3)����������Ҫ����AlCl3��FeCl3�����������Al����������___________ _____________________________________________________________��
(4)Ϊ�ⶨ�Ƶõ���ˮAlCl3��Ʒ(������FeCl3)�Ĵ��ȣ���ȡ16.25 g��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�У����˳�����������ᆳϴ�ӡ����ա���ȴ�����أ���������Ϊ0.32 g��
��д���������ӹ������漰�����ӷ���ʽ��__________________________��__________________________��
��AlCl3��Ʒ�Ĵ���Ϊ__________��
(5)��ҵ����һ��������Ϊԭ���Ʊ���ˮAlCl3�Ĺ����У����һ������AlCl3��6H2O��ˮ�Ʊ���ˮAlCl3��ʵ����һ���ķ�����__________________ ______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Na2O2��NaHCO3��Ϻ����ܱ������м��ȳ�ַ�Ӧ���ų����壬��ȴ���й�������ʣ�࣬����ѡ���ȷ���� (����)��
| Na2O2/mol | NaHCO3/mol | ʣ��Ĺ������� | |
| A | 1 | 2 | Na2CO3 |
| B | 1.5 | 2 | Na2O2��Na2CO3 |
| C | 2 | 1 | Na2O2��NaOH��Na2CO3 |
| D | 2 | 2 | NaOH��Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(NH4)2PtCl6�������ȷֽ⣬���ɵ������Ȼ��⡢�Ȼ�狀ͽ��������ڴ˷ֽⷴӦ�У����������뻹ԭ��������ʵ���֮���� (����)��
A��2��3 B��3��2
C��4��3 D��1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��SO2ͨ������Fe2(SO4)3��Һ�У���ȫ��Ӧ���ټ���K2Cr2O7��Һ��������������ѧ��ӦΪSO2��2Fe3����2H2O===SO ��2Fe2����4W���٣�Cr2O
��2Fe2����4W���٣�Cr2O ��aFe2����bH���D��Cr3����Fe3����H2O���ڡ������й�˵����ȷ���� (����)��
��aFe2����bH���D��Cr3����Fe3����H2O���ڡ������й�˵����ȷ���� (����)��
A����ԭ�ԣ�Cr3����SO2
B������ʽ���У�a��6��b��7
C��Cr2O �ܽ�Na2SO3������Na2SO4
�ܽ�Na2SO3������Na2SO4
D������ʽ����WΪOH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��̬��������������������ܶ�Ϊ27��ȡ0.54 g����ǡ����Ũ��Ϊ0.2 mol��L��1����ˮ100 mL��ȫ��Ӧ��ʹ��ˮ��ȫ��ɫ��������ķ���ʽΪ(����)
A��C4H6 B��C4H8 C��C4H10 D������ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ��þ�ĺϽ�4��6g��ȫ����Ũ���ᣬ����Ӧ�����ᱻ��ԭֻ����4480mL��NO2�����336mL��N2O4���壨�������㵽��״�������ڷ�Ӧ�����Һ�У���������������������Һ�����ɳ���������Ϊ
A��9��02g B��8��51g C��8��26g D��7��04g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com