��2013?����һģ����ҵ����K
3[Fe��C
2O
4��
3]?3H
2O�Ǵ���ɫ���壬��421��553��ʱ���ֽ�ΪFe
2O
3��K
2CO
3��CO��CO
2��H
2O��ʵ�����ɲ����������壨FeC
2O
4?2H
2O��������أ�K
2C
2O
4�������ᣨH
2C
2O
4����˫��ˮ��H
2O
2������Ʊ���
��ش��������⣺
��1���H
2O
2�ĵ���ʽ
��[Fe��C
2O
4��
3]
3-��������
[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�����������������
[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�����������������
��
��2����ƽ�����ܷ�Ӧ����ʽ��
2
2
FeC
2O
4?2H
2O+
1
1
H
2O
2+
3
3
K
2C
2O
4+
1
1
H
2C
2O
4=
3
3
K
3[Fe��C
2O
4��
3]?3H
2O
��3���Ʊ�������Ҫ��ֹ���ᱻH
2O
2��������д�����ᱻH
2O
2�����Ļ�ѧ��Ӧ����ʽ
H2C2O4+H2O2=2CO2��+2H2O
H2C2O4+H2O2=2CO2��+2H2O
��
��4���������ȶ��Կ������ȶ�����K����������Cu
2++4NH
3?[Cu��NH
3��
4]
2+�����ȶ���������ʽΪ
k=����֪K[Fe��C
2O
4��
3]
3-=10
20��K[Fe��SCN��
3]=2��10
3���ܷ���KSCN��Һ����K
3[Fe��C
2O
4��
3]?3H
2O�е���Ԫ�أ�
��
��
����ǡ�������ѡ��������Ƽ�����Ԫ�صķ���
ȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮����
ȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮����
��
��5����Ԫ�ؿ����γɶ�����������һ����������A���������ʳƷ���Ӽ�������ɷ���A�н���K��Fe��C��N����Ԫ�أ�ȡ36.8gA������400�棬�ֽ��KCN��Fe
3C��C��N
2�����ɵĵ����ۺϳɱ�״���µ����Ϊ2.24L��Fe
3C������C������3����Fe
3C�����ʵ����ǵ������ʵ�����
����A�Ļ�ѧʽΪ
K4Fe��CN��6
K4Fe��CN��6
��



 ��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ��������������������ʴ�Ϊ��
��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ��������������������ʴ�Ϊ�� ��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�������������������
��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�������������������


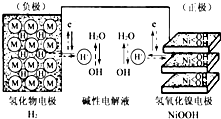 ��2013?����һģ���������г�ʹ�ÿɳ���أ�����ʾ��ͼ��ͼ���⻯��缫Ϊ����������ɿ���H2ֱ�Ӳμӷ�Ӧ��������̫���ܷ��巢�磬��һ���ֵ����������������ҹ�������ع��磮����˵����ȷ���ǣ�������
��2013?����һģ���������г�ʹ�ÿɳ���أ�����ʾ��ͼ��ͼ���⻯��缫Ϊ����������ɿ���H2ֱ�Ӳμӷ�Ӧ��������̫���ܷ��巢�磬��һ���ֵ����������������ҹ�������ع��磮����˵����ȷ���ǣ�������