
| A£®¼ÓČėAgNO3ČÜŅŗÉś³ÉµÄ°×É«³Įµķ²»ČÜÓŚĻ”ŃĪĖį£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠCl£“ęŌŚ |
| B£®¼ÓČė°±Ė®Ź±Éś³É°×É«³Įµķ£¬µ±°±Ė®¹żĮæŹ±°×É«³ĮµķĻūŹ§£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠAl3+“ęŌŚ |
| C£®¼ÓČėNaOHČÜŅŗ²¢¼ÓČČ£¬ÓŠÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢåÉś³É£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠNH4+“ęŌŚ |
| D£®¼ÓČėŃĪĖįÓŠÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢåÉś³É£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠ“óĮæCO32£“ęŌŚ |

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ±ąŗÅ | ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ¢Ł | | |
| ¢Ś | | |
| ¢Ū | | |
| ¢Ü | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
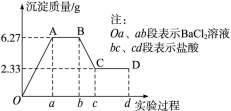
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ÓČėAgNO3ČÜŅŗÉś³ÉµÄ°×É«³Įµķ²»ČÜÓŚĻ”ŃĪĖį£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠCl£“ęŌŚ |
| B£®¼ÓČė°±Ė®Ź±Éś³É°×É«³Įµķ£¬µ±°±Ė®¹żĮæŹ±°×É«³ĮµķĻūŹ§£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠAl3+“ęŌŚ |
| C£®¼ÓČėNaOHČÜŅŗ²¢¼ÓČČ£¬ÓŠÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢåÉś³É£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠNH4+“ęŌŚ |
| D£®¼ÓČėŃĪĖįÓŠÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢåÉś³É£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠ“óĮæCO32£“ęŌŚ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
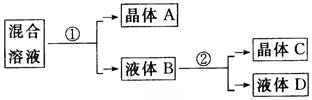
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®NaOH | B£®NH3”¤H2O | C£®ZnCO3 | D£®Fe2O3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com