�����������ķ�չ���������No
x���ѳ�Ϊ�����е���Ҫ��̬��Ⱦ��֮һ��
��1�������ٷɻ��ŷŵ�β����ƽ������NO����Ҫ��Դ�������ƻ�������Ļ���Ϊ��
��O
3O+O
2 ��NO��NO
2+O
2��NO
2+O��NO+O
2������Ӧ���ܷ�ӦʽΪ
������
NO
NO
�Ǵ�����

��2������β���к���NO
x��CO��̼�⻯����ȣ�
��β������װ����װ�к�Pd�ȹ���Ԫ�صĴ����������ڴ�������������������õĻ�����ͼ1��ʾ��β�����������з������ܷ�Ӧ��ѧ����ʽ��
��
��NO
x��̼�⻯���������������·����⻯ѧ��Ӧ����������Ⱦ�������Ϊ���⻯ѧ����������ѧ�߶�ij���У����й⻯ѧ�����ı仯������вⶨ��ʵ������ͼ2����ͼ���֪��������Ⱦ����
ȩ��O3
ȩ��O3
�ȣ�ȩ��O
3�ķ�ֵ������14��00���ҵ���Ҫԭ����
��ʱ�չ�ǿ�ң��⻯ѧ��Ӧ�������
��ʱ�չ�ǿ�ң��⻯ѧ��Ӧ�������
��
�۲ⶨ����β����No
x�ķ���֮һ����3%��H
2O
2��Һ����β���е�NO
x����HNO
3������NaOH����Һ�ζ�HNO
3����Ҫ�ⶨ��״����β����NO
x����������������������
ACD
ACD
��ѡ����ţ���
A������β��������� B�����ֵ�������������
C��NaOH����Һ�����ʵ���Ũ�� D��������NaOH����Һ�����
E���ӵζ���ʼ��ָʾ����ɫ�����ʱ��
��3�����Ṥҵβ���е�NO
x���ô�����Һ���գ��йصĻ�ѧ��ӦΪ��
2NO
2+Na
2O=NaNO
3+CO
2NO+NO
2+Na
2CO
3=2NaNO
3+CO
2����0.5L 2mol/L�Ĵ�����Һǡ������һ�������Ṥҵβ���е�NO
x������Һ��������44g����C0
2ȫ���ų�������NO
2��NO�������Ϊ
7��1
7��1
��





 ����������ᷢչ�����������ѳ�Ϊ������Ṳͬ��ע�Ľ��㣮
����������ᷢչ�����������ѳ�Ϊ������Ṳͬ��ע�Ľ��㣮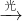 O+O2
O+O2 
 O��O2 ��NO��O3��NO2��O2 ��NO2��O��NO��O2
O��O2 ��NO��O3��NO2��O2 ��NO2��O��NO��O2 

