



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®1 mol H2OµÄÖŹĮæŹĒ18 g/mol |
| B£®CH4µÄĦ¶ūÖŹĮæĪŖ16g |
| C£®3.01”Į1023øöSO2·Ö×ÓµÄÖŹĮæŹĒ32 g |
| D£®±ź×¼×“æöĻĀ£¬1 molČĪŗĪĪļÖŹĢå»ż¾łĪŖ22.4 L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®2.4g Mg2+Ėłŗ¬µē×ÓŹżÄæĪŖ1.2NA |
| B£®±ź×¼×“æöĻĀ22.4L CCl4Ėłŗ¬·Ö×ÓŹżÄæĪŖNA |
| C£®VmĢå»żCH4Ėłŗ¬ÖŹ×ÓŹżÄæĪŖ10NA |
| D£®18g NH4+Ėłŗ¬µē×ÓŹżÄæĪŖ11 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®1£ŗ1 | B£®1£ŗ2 | C£®1£ŗ3 | D£®3£ŗ2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®·Ö×ÓŹż | B£®Ō×ÓŹż | C£®µē×ÓŹż | D£®ÖŹĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
 MnCl2+C12ӟ+2H2O
MnCl2+C12”ü+2H2O²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā

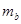 £ØĢī”°”±<”¢”°>”±”¢»ņ”°=”±£©”£
£ØĢī”°”±<”¢”°>”±”¢»ņ”°=”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®±ź×¼×“æöĻĀ£¬22.4L H2Oŗ¬ÓŠµÄ·Ö×ÓŹżĪŖ NA |
| B£®³£ĪĀ³£Ń¹ĻĀ£¬1.06g Na2CO3ČÜÓŚĖ®ŠĪ³ÉµÄČÜŅŗÖŠŗ¬ÓŠNa+Ąė×ÓŹżĪŖ0.02 NA |
| C£®Ķس£×“æöĻĀ£¬NAøöCO2·Ö×ÓÕ¼ÓŠµÄĢå»żĪŖ22.4L |
| D£®ĪļÖŹµÄĮæÅضČĪŖ0.5 mol/LµÄMgCl2ČÜŅŗÖŠ£¬ŗ¬ÓŠCl-øöŹżĪŖNA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com