
·ÖĪö £Ø1£©NH4ClŹĒĒæĖįČõ¼īŃĪ£¬Ė®½āĻŌĖįŠŌ£»øł¾ŻµēŗÉŹŲŗćÅŠ¶ĻĄė×ÓÅØ¶Č“óŠ”£»
£Ø2£©Na2SĖ®ČÜŅŗÖŠ·Ö²½Ė®½ā£¬ŅŌµŚŅ»²½Ė®½āĪŖÖ÷£¬Ķ¬Ź±“ęŌŚHS-?H++S2-£»
£Ø3£©NaHCO3ĪŖĒæ¼īČõĖįŃĪ£¬Ė®½ā³Ź¼īŠŌ£¬ĮņĖįĀĮČÜŅŗÖŠĀĮĄė×ÓĖ®½ā³ÉĖįŠŌ£»ĮņĖįĀĮŗĶĢ¼ĖįĒāÄĘĖ®ČÜŅŗÖŠ·¢ÉśĖ«Ė®½āÉś³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶ¶žŃõ»ÆĢ¼£¬NaHCO3ČÜŅŗÓėNaAlO2ČÜŅŗ»ģŗĻ²śÉś°×É«³ĮµķĒāŃõ»ÆĀĮ£®
½ā“š ½ā£ŗ£Ø1£©ļ§øłĄė×ÓĖ®½ā£¬Ź¹ČÜŅŗ³ŹĖįŠŌ£¬¼“pH£¼7£¬ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĪŖNH4++H2O=NH3•H2O+H+£¬Ņ»°ćĒéæöĻĀŃĪµÄĖ®½ā³Ģ¶Č¶¼±Č½ĻŠ”£¬ĖłŅŌČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”Ė³ŠņĪŖc£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£¬
¹Ź“š°øĪŖ£ŗ£¼£»c£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£»
£Ø2£©Na2SŅŌµŚŅ»²½Ė®½ā£¬Na2SµÄĖ®½ā³Ģ¶Č“óÓŚNaHS£¬ĖłŅŌNa2SµÄPH“óÓŚNaHS£¬Ė®½āĄė×Ó·½³ĢŹ½ĪŖ£ŗS2-+H2O?HS-+OH-£¬HS-+H2O?H2S+OH-£¬ČÜŅŗÖŠ»¹“ęŌŚHS-?H++S2-£¬ĖłŅŌĮ½ÖÖČÜŅŗÖŠĪ¢Į£ÖÖĄąĻąĶ¬£¬
¹Ź“š°øĪŖ£ŗ£¾£»=£»
£Ø3£©Ģ¼ĖįĒāÄĘČÜŅŗÖŠ£¬Ģ¼ĖįĒāøłĄė×Ó²æ·ÖĖ®½ā£ŗHCO3-+H2O?H2CO3+OH-£¬ŌņČÜŅŗĻŌŹ¾¼īŠŌ£¬ĮņĖįĀĮČÜŅŗÖŠĀĮĄė×ÓĖ®½ā³ÉĖįŠŌ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAl3++3H2O?Al£ØOH£©3+3H+£¬ĮņĖįĀĮŗĶĢ¼ĖįĒāÄĘĖ®ČÜŅŗÖŠ·¢ÉśĖ«Ė®½āÉś³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶ¶žŃõ»ÆĢ¼£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAl3++3HCO3-=Al£ØOH£©3”ż+3CO2”ü£¬NaHCO3ČÜŅŗÓėNaAlO2ČÜŅŗ»ģŗĻ²śÉś°×É«³ĮµķĒāŃõ»ÆĀĮŗĶĢ¼ĖįøłĄė×Ó£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗAlO2-+HCO3-+H2OØTAl£ØOH£©3”ż+CO32-£¬
¹Ź“š°øĪŖ£ŗHCO3-+H2O?H2CO3+OH-£»Al3++3H2O?Al£ØOH£©3+3H+£»ÓŠ°×É«³ĮµķŗĶĪŽÉ«ĘųĢå²śÉś£»Al3++3HCO3-=Al£ØOH£©3”ż+3CO2”ü£»ÓŠ°×É«³Įµķ²śÉś£»HCO3-+AlO2-+H2O=Al£ØOH£©3”ż+CO32-£®
µćĘĄ øĆĢāŹĒ»ł“”ŠŌŹŌĢāµÄ漲飬ŹŌĢāÄŃŅ׏ŹÖŠ£¬»ł“”ŠŌĒ森øĆĢāµÄ¹Ų¼üŹĒĆ÷Č·ŃĪĄąĖ®½āµÄŌĄķ£¬Č»ŗó½įŗĻĢįøߊÅĻ¢Įé»īŌĖÓĆ¼“æÉ£¬ÓŠĄūÓŚÅąŃųѧɜµÄĮé»īÓ¦±äÄÜĮ¦£¬“šĢāŹ±×¢ŅāĄė×Ó·“Ó¦·½³ĢŹ½µÄÕżČ·ŹéŠ“£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ŌŖĖŲ | o | p | |
| µēĄėÄÜkJ•mol-1 | I1 | 717 | 759 |
| I2 | 1 509 | 1 561 | |
| I3 | 3 248 | 2 957 | |
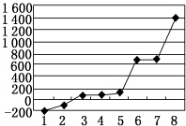
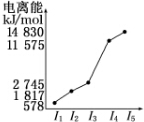
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¶¼ÄÜČÜÓŚÅØŃĪĖį»ņÅØĻõĖįÖŠ | |
| B£® | ¶¼ÄÜČÜÓŚĒāŃõ»ÆÄĘČÜŅŗÖŠ | |
| C£® | ĖüĆĒµÄČŪµć¶¼ŗÜøߣ¬³£ÓĆ×öÄĶ»š²ÄĮĻ | |
| D£® | ³£ĪĀĻĀ¶¼²»ÄÜÓėĖ®·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀĻĀ£¬1L0.1mol•L-1µÄNH4NO3ČÜŅŗÖŠµŖŌ×ÓŹżĪŖ0.2NA | |
| B£® | 1molōĒ»łÖŠµē×ÓŹżĪŖ10 NA | |
| C£® | ŌŚ·“Ó¦KIO3+6HIØTKI+3I2+3H2OÖŠ£¬ĆæÉś³É3 mol I2×ŖŅʵĵē×ÓŹżĪŖ6 NA | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬22.4LŅŅĻ©ÖŠC--H¼üŹżĪŖ4 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 »Æѧ·“Ó¦ŌĄķŌŚæĘŃŠŗĶÉś²śÖŠÓŠ¹ć·ŗÓ¦ÓĆ£ŗ
»Æѧ·“Ó¦ŌĄķŌŚæĘŃŠŗĶÉś²śÖŠÓŠ¹ć·ŗÓ¦ÓĆ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 100 L | B£® | 10 L | C£® | 11.2L | D£® | 44.8L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 5.6L | B£® | 11.2L | C£® | 22.4L | D£® | 33.6L |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com