I����һ�����������ۼ��뵽װ��100mLijŨ�ȵ�ϡ������Һ�г�ַ�Ӧ��
��1��������ʣ��mg�����ۣ��ռ���NO����448mL����״���£���
�� ������Һ�е����ʵĻ�ѧʽ��____________________��
�� ԭ������Һ�����ʵ���Ũ��Ϊ��____________________mol/L
��2����������-Һ���������μ���ϡ����ֱ���պò��ٲ�������Ϊֹ����������������ɺ���ɫ����ʱ������������ng��
�ٴ�ʱ��Һ�����ʵĻ�ѧʽ��__________________��
��m-n��ֵΪ_______________g��
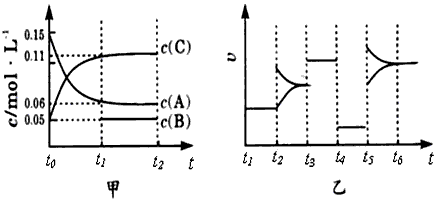
����ij�ܱ������м���0.3molA��0.1molC��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��ı仯����ͼ�м�ͼ��ʾ����ͼ����ͼΪt2ʱ�̺�ı�������������ƽ����ϵ�з�Ӧ������ʱ��仯����������ĸ��ζ����ı�һ�ֲ�ͬ��������������������ͬ����֪t3 ~t4��Ϊʹ�ô���
����֪t0~ t1�� c ( B)δ�����ݡ�
��3����t1=15s ����t0~t1����C Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ____________________��
��4��t4~t5�θı������Ϊ_______________��B����ʼ���ʵ���Ϊ___________________��
��5��t5~t6��������A�����ʵ���������0.03mol �����˹����������������Ƚ�������ΪakJ��д���÷�Ӧ���Ȼ�ѧ����ʽ____________________________��