
���� ��1��������ϡ��ǰ�����ʵ��������������Ҫ10%����������Һ������������V=$\frac{m}{��}$������Һ�������ȷ������ˮ�������
��������Ҫ10%����������Һ�����ѡ����Ͳ���
��2��������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�����ϡ��ǰ�����ʵ����ʵ������������ҪŨ����������
����������һ�����ʵ���Ũ����Һ��һ�㲽����
�۷������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��������Ҫ10%����������Һ������Ϊm������ϡ��ǰ�����ʵ��������䣬m��10%=27.5g��2%�����m=5.5g����Һ�����V=$\frac{m}{��}$=$\frac{5.5g}{1.00g•c{m}^{-3}}$=5.4mL����Ҫˮ������Ϊ27.5g-5.5g=22g��ˮ���ܶ�Ϊ1g/mL��������Ҫˮ�����Ϊ22.0mL��
�ʴ�Ϊ��5.5g��22ml��
��ͨ���ټ����֪��Ҫ10%����������Һ���Ϊ5.4mL������Ӧѡ��10mL��Ͳ��
�ʴ�Ϊ��10ml��
��2����98%���ܶ�Ϊ1.84g•cm-3����Ũ��������ʵ���Ũ��C=$\frac{1000��1.84��98%}{98}$=18.4mol/L������ҪŨ�������ΪV������ϡ��ǰ�����ʵ����ʵ����������ã�V��18.4mol/L=3mol•L-1��100mL�����V=16.3mL��
�ʴ�Ϊ��16.3��
����Ũ��Һ����һ�����ʵ���Ũ��ϡ��Һһ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ��˳��Ϊ��ADECBHGF��
�ʴ�Ϊ��ADECBHGF��
���������C�����а�δ��ȴ��ϡ����ע������ƿ�У���ȴ��Һ���½�����Һ���ƫС����ҺŨ��ƫ�ߣ�
�������D��������ȡŨ�������Ͳ��������Ũ��������������ˮϴ�Ӳ���ϴ��Һת��E�����е�С�ձ��У�������ȡ��Ũ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ�
�������D������Ŀ�⸩�ӣ�������ȡ��Ũ������Һ���ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ߣ�ƫ�ͣ�
���� ���⿼��������һ�������ٷ���Ũ�Ⱥ�һ�����ʵ���Ũ����Һ����ȷ���õ�ԭ������ȷ�IJ��������ǽ���ؼ������ؿ���ѧ���Ի���ʵ��ԭ���Ͳ����������������Ŀ�ѶȲ���


 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO2��SO2��BF3��NCl3������û��һ��������ԭ�ӵ��������Ӷ�������8�����ȶ��ṹ | |
| B�� | ���ʵľ�����һ�������ڵ����������� | |
| C�� | P4��CH4����������������Ҽ��Ƕ�Ϊ109��28�@ | |
| D�� | NaCl��������ÿ��Na+��������������Cl-����12�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����£�ȼ��1mol S�ų�������Ϊ297.23 kJ | |
| B�� | S��g��+O2��g���TSO2��g���ų�����������297.23 kJ | |
| C�� | S��g��+O2��g���TSO2��g���ų�������С��297.23 kJ | |
| D�� | �γ�1molSO2�Ļ�ѧ�����ͷŵ����������ڶ���1molS��s����1molO2��g���Ļ�ѧ�������յ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��0.10mol•L-1CH3COONa��Һ��ͨHCl��c��Na+����c��CH3COOH��=c��Cl-�� | |
| B�� | ��0.10mol•L-1NH4HCO3��Һ��ͨCO2��c��NH4+���Tc��HCO3-��+c��CO32-�� | |
| C�� | ��0.10mol•L-1NaHSO3��Һ��ͨNH3��c��Na+����c��NH4+����c��SO32-�� | |
| D�� | ��0.10mol•L-1 Na2SO3��Һ��ͨSO2��c��Na+���T2[c��HSO3-��+c��SO32-��+c��H2SO3��] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �е��ʲμӻ��е������ɵĻ�ѧ��Ӧһ����������ԭ��Ӧ | |
| B�� | �е������ɵķֽⷴӦ����������ԭ��Ӧ����Ϊ������Ԫ�ػ��ϼ۱�Ȼ�����仯 | |
| C�� | û�е��ʲμӵĻ��Ϸ�Ӧһ���Ƿ�������ԭ��Ӧ | |
| D�� | ������ԭ��Ӧ����һ��Ԫ�ر�����ʱ��һ������һ��Ԫ�ر���ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
 ��
��
 ��
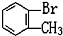
��
 ��
��
 ��
��
 ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com