
��14�֣�ij�о���ѧ��С��������ϵ�֪��Ư����������Һ��Ӧ����ȡ��������ѧ����ʽΪ��Ca(ClO)2��CaCl2��2H2SO42CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣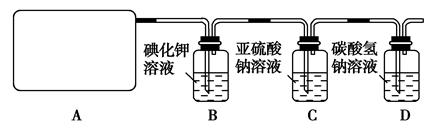
�Իش�
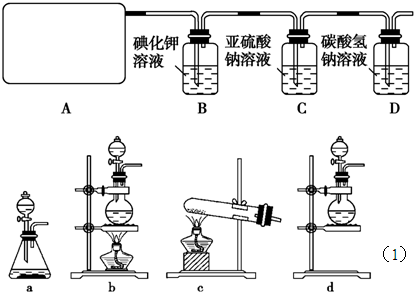
��1����ʵ����A���ֵ�װ����________(��дװ�õ����)��
��2��B�з�Ӧ�Ļ�ѧ����ʽ�� ��
��3��д��C�з�Ӧ�����ӷ���ʽ ����������С��ͬѧ���һ��ʵ��,֤��ϴ��ƿC�е�Na2SO3�ѱ�����(����ʵ�鲽��)��_________________ ��
��4��д����Dװ���з�����Ӧ�����ӷ���ʽ ��
��5����ʵ��������Ե�ȱ����__________________________________��
��6����С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ�����������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol��L��1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ2Na2S2O3��I2===Na2S4O6��2NaI����Ӧ���ʱ��������Na2S2O3 200 mL�����Ư����Ca(ClO)2����������Ϊ___ _____��
��1��b ��2��Cl2+2KI=2KCl+I2 ��3��Cl2+SO32-+H2O=SO42-+2Cl-+2H+��ȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�������
��4��Cl2+H2O H++Cl-+HClO ��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2 ��+Cl-+HClO )����5����β������װ�ã���6��35.8%(��35.75%)��
H++Cl-+HClO ��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2 ��+Cl-+HClO )����5����β������װ�ã���6��35.8%(��35.75%)��
������1�������Һ���ϼ��ȣ�ѡb ��2��Cl2+2KI=2KCl+I2 ��3��Cl2+SO32-+H2O=SO42-+2Cl-+2H+��SO32�D����SO42�D�ļ��飬Ӧ�ȳ�ȥ����ƣ�ȡ������Ӧ�����Һ���Թ��У�����HCl��Һ�����ٲ�������Ϊֹ���ٵμ�BaCl2��Һ������а�ɫ�������ɣ�֤��Na2SO3�ѱ�������
��4��Cl2+H2O H++Cl-+HClO ��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2 ��+Cl-+HClO )����5����β������װ�ã���6��35.8%(��35.75%)��
H++Cl-+HClO ��HCO3-+H+=H2O+CO2��������Cl2+ HCO3-=CO2 ��+Cl-+HClO )����5����β������װ�ã���6��35.8%(��35.75%)��
����������������0.1mol��L��1��0.02L=0.002mol��
2Na2S2O3+I2=Na2S4O6+2NaI
2 1
0.002 n1
n1=0.001
���ⵥ����0.001mol��
ClO��+2I��+2H��Cl��+I2+H2O
1 2
n2 0.001
n2=0.0005
�����������0.0005mol����ô������Ƶ��������ǣ�0.0005mol��143g��mol��1=0.0715g��������Щ��������Ǵ�250mL��ȡ����25mL�еģ�������Ʒ�д�����Ƶ�������0.0715g��10=0.715g����ôƯ���д�����Ƶ������������ǣ�0.715g/2.0g��100%=35.75%��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ij�о���ѧ��С��������ϵ�֪��Ư����������Һ��Ӧ����ȡ��������ѧ����ʽΪ��Ca(ClO)2��CaCl2��2H2SO42CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
�Իش�
��1����ʵ����A���ֵ�װ����________(��дװ�õ����)��
��2��B�з�Ӧ�Ļ�ѧ����ʽ�� ��
��3��д��C�з�Ӧ�����ӷ���ʽ ����������С��ͬѧ���һ��ʵ��,֤��ϴ��ƿC�е�Na2SO3�ѱ�����(����ʵ�鲽��)��_________________ ��
��4��д����Dװ���з�����Ӧ�����ӷ���ʽ ��
��5����ʵ��������Ե�ȱ����__________________________________��
��6����С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ�����������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol��L��1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ2Na2S2O3��I2===Na2S4O6��2NaI����Ӧ���ʱ��������Na2S2O3200 mL�����Ư����Ca(ClO)2����������Ϊ___ _____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ����Ͽ�и�����ѧ�ڵ��п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ij�о���ѧ��С��������ϵ�֪��Ư����������Һ��Ӧ����ȡ��������ѧ����ʽΪ��Ca(ClO)2��CaC l2��2H2SO4
l2��2H2SO4 2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣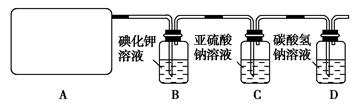
�Իش�
��1����ʵ����A���ֵ�װ����________(��дװ�õ����)��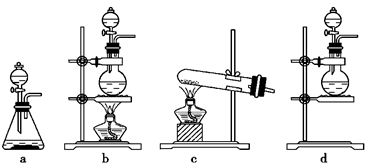
��2��B�з�Ӧ�Ļ�ѧ����ʽ�� ��
��3��д��C�з�Ӧ�����ӷ���ʽ ����������С��ͬѧ���һ��ʵ��,֤��ϴ��ƿC�е�Na2SO3�ѱ�����(����ʵ�鲽��)��_ ________________ ��
________________ ��
��4��д����Dװ���з�����Ӧ�����ӷ���ʽ ��
��5����ʵ��������Ե�ȱ����__________________________________��
��6����С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ�����������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol��L��1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ2Na2S2O3��I2===Na2S4O6��2NaI����Ӧ���ʱ��������Na2S2O3 200 mL�����Ư����Ca(ClO)2����������Ϊ___ _____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����и����ڶ���������⻯ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ij�о���ѧ��С��������ϵ�֪��Ư����������Һ��Ӧ����ȡ��������ѧ����ʽΪ��Ca(ClO)2��CaCl2��2H2SO42CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
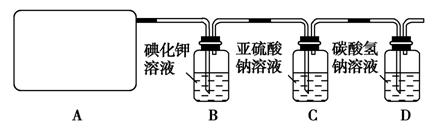
�Իش�
��1����ʵ����A���ֵ�װ����________(��дװ�õ����)��

��2��B�з�Ӧ�Ļ�ѧ����ʽ�� ��
��3��д��C�з�Ӧ�����ӷ���ʽ ����������С��ͬѧ���һ��ʵ��,֤��ϴ��ƿC�е�Na2SO3�ѱ�����(����ʵ�鲽��)��_________________ ��
��4��д����Dװ���з�����Ӧ�����ӷ���ʽ ��
��5����ʵ��������Ե�ȱ����__________________________________��
��6����С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ�����������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol��L��1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ2Na2S2O3��I2===Na2S4O6��2NaI����Ӧ���ʱ��������Na2S2O3 200 mL�����Ư����Ca(ClO)2����������Ϊ___ _____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com