
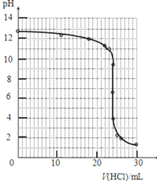
| V��HCl��/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
| ָʾ�� | ��ɫ��Χ ��pH�� |
����Χ����ɫ | ||
| ǰ | �м� | �� | ||
| ���� | 3.1��4.4 | �� | ��ɫ | �� |
| ʯ�� | 5.0��8.0 | �� | ��ɫ | �� |
| ��̪ | 8.2��10.0 | �� | �ۺ� | �� |
 ��
�� ��
��| 0.1000mol/L��0.024L |
| 0.02L |
| 4.800g |
| 5.000g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��ԭ�ر�����ѧ��һ�������¿���ѧ�Ծ����������� ���ͣ������
(16��) ijУ��ѧ��ȤС���ͬѧ���������ϵ�֪�����������ܵ�����Ĥ��ʹ��������Χ�Ľ���(������ˮ��)����������ֵ������������Բ�������ʢ�ź������̲ˡ�Ϊ�˸�С���ͬѧ�������������Ĥ������̽������������£������������ա�
(1)������ǯ��סһ���ȥ����Ĥ����Ƭ�����ھƾ��ƻ��������գ���Ƭ����Ӵ�����IJ��ֱ䰵��Ƭ�̺����������ҡ����������ҡ�Σ�ȴ���������������䡣������Ϊ����������Ĥ���۵�________(����ڡ����ڡ�)�ڲ������۵㣬�������ס�����Բ�������������
(2)ȡ����������������סһС������ƺ����ˮ�������������������ЩС���ٰ���סһС������ƺ����ˮ�У�Ѹ�پ��д��������ݲ������Խ������е�ԭ��д����صĻ�ѧ����ʽ��___________ ___________________��
(3)��ɰֽ��ĥһ��Ƭ��ʹ������ֲڣ��ٽ������CuSO4ϡ��Һ�У�2��3 min����������ɫ���帽��������档��д��������ɫ��������ӷ���ʽ�� ��
(4)������δ��ɰֽ��ĥ������Ƭ����������������Һ�У�Ƭ�̺�����������ɫ���塣��д����������Ĥ������������Һ��Ӧ�����ӷ���ʽ��___________________________��
(5)��ȡһ���������ޣ��ռ���һ��CO2�����������Ũ����������Һ�������ѿڷ�ա����Է��������ޡ����ǡ����죬������ˣ���һ����������ֻ����첢���������Խ���Ϊʲô��д���йص����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��һ�������¿���ѧ�Ծ��������棩 ���ͣ������
(16��) ijУ��ѧ��ȤС���ͬѧ���������ϵ�֪�����������ܵ�����Ĥ��ʹ��������Χ�Ľ���(������ˮ��)����������ֵ������������Բ�������ʢ�ź������̲ˡ�Ϊ�˸�С���ͬѧ�������������Ĥ������̽������������£������������ա�
(1)������ǯ��סһ���ȥ����Ĥ����Ƭ�����ھƾ��ƻ��������գ���Ƭ����Ӵ�����IJ��ֱ䰵��Ƭ�̺����������ҡ����������ҡ�Σ�ȴ���������������䡣������Ϊ����������Ĥ���۵�________(����ڡ����ڡ�)�ڲ������۵㣬�������ס�����Բ�������������
(2)ȡ����������������סһС������ƺ����ˮ�������������������ЩС���ٰ���סһС������ƺ����ˮ�У�Ѹ�پ��д��������ݲ������Խ������е�ԭ��д����صĻ�ѧ����ʽ��___________ ___________________��
(3)��ɰֽ��ĥһ��Ƭ��ʹ������ֲڣ��ٽ������CuSO4ϡ��Һ�У�2��3 min����������ɫ���帽��������档��д��������ɫ��������ӷ���ʽ�� ��
(4)������δ��ɰֽ��ĥ������Ƭ����������������Һ�У�Ƭ�̺�����������ɫ���塣��д����������Ĥ������������Һ��Ӧ�����ӷ���ʽ��___________________________��
(5)��ȡһ���������ޣ��ռ���һ��CO2�����������Ũ����������Һ�������ѿڷ�ա����Է��������ޡ����ǡ����죬������ˣ���һ����������ֻ����첢���������Խ���Ϊʲô��д���йص����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡģ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com