
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2������n=cv��������Na2CO3�����ʵ���������Na2CO3•10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3•10H2O��������
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��4������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1������ƿ�Ĺ��û��480mL��ֻ����500mL����ƿ������0.2mol/L��Na2CO3��Һ500mL�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ��ʻ�ȱ��500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500 mL����ƿ����ͷ�ιܣ�
��2��ʵ������Ҫ0.2mol/L��Na2CO3��Һ480mL����������ƿ�Ĺ��û��480mL��ֻ����500mL����ƿ����500mLNa2CO3��Һ��ҪNa2CO3�����ʵ���Ϊ��0.5L��0.2mol/L=0.1mol��Na2CO3•10H2O�����ʵ���Ϊ0.1mol��Na2CO3•10H2O������Ϊ��0.1mol��286g/mol=28.6g��
�ʴ�Ϊ��28.6��
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳���Ǣݢ٢ܢޢۢޢߢڣ�
�ʴ�Ϊ���ݢ٢ܢޢۢޢߢڣ�
��4����̼����ʧȥ�˲��ֽᾧˮ�����³������ʵ����������������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���
���á���������ij��������������嵼�³������ʵ�����ƫС���������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
��̼���ƾ��岻�������л����Ȼ��ƣ�����̼���Ƶ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
�ܳ���̼���ƾ���ʱ�����������⣬���³������ʵ����������������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���
������ƿδ�������ʹ�ò�Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ����������Զ����Ƶ���ҺŨ����Ӱ�죻
��ѡ���٢ܣ��ݣ�
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ��״����Ǽ������ʵ��������ܶ�ͬѧ����Һ�������Ϊ��480mL�����³�������Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | A��B���γ�AB32-��A2B42-������ | |
| B�� | B���⻯��ķе����C���⻯��ķе� | |
| C�� | ����C�ľ����к��й��ۼ��ͷ��»��� | |
| D�� | ��A��D�γɵ�һ�����ʿ�������ȡ��ˮ�еⵥ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �������� | ��ѧ��Ӧ | ��Ӧ��� |
| ����������� | 4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2 | 4%����Ԫ����ʧ������¯�� |
| ������ | 2SO2+O2$?_{��}^{����}$2SO3 | SO2ת����Ϊ90% |
| SO3������ | SO3+H2O=H2SO4 | SO3������Ϊ100% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2��SO3���Ԫ����ͬ����H2O��Ӧ����Ҳ��ͬ | |
| B�� | Ũ��������ֽ⣬����ʱ��ʵ���ҿ�����Ũ����ʻ�ɫ | |
| C�� | CO��NO��NO2�����γɹ⻯ѧ�������Ǵ�����Ⱦ���壬�ڿ����ж����ȶ����� | |
| D�� | ������ˮ�����ԣ���������ˮ�еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ |
�鿴�𰸺ͽ���>>
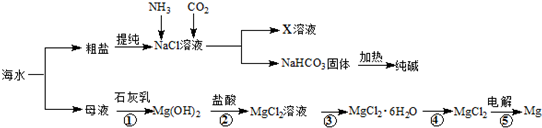
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
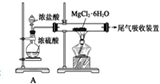 ������װ��A���������Ʊ������HCl����
������װ��A���������Ʊ������HCl�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol/L�� Fe2��SO4��3��Һ�к���2NA��Fe3+��������ˮ�⣩ | |
| B�� | 1mol�� Fe2��SO4��3��S2-��Ӧ��ת��2NA������ | |
| C�� | �ڸ���Һ�У�K+��NH4+��I-��SO42-���Դ������� | |
| D�� | ��Cu��Ӧ�����ӷ���ʽΪ��Fe3++Cu�TFe2++Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ӻ�����һ���������ۼ� | |
| B�� | ���ۻ�����һ���������Ӽ� | |
| C�� | ��̬���ʵķ�����һ�����ڹ��ۼ� | |
| D�� | �ǽ���Ԫ�صĻ�����һ���������Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
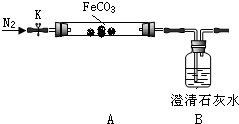
| ʵ�鲽�� | Ԥ������ͽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �Լ� | FeCl3��Һ | ����KMnO4��Һ | NaHCO3��Һ |
| ���� | ��Һ����ɫ | ��ɫ | �ų����� |
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com