
·ÖĪö £Ø1£©ĀĮĄė×Ó”¢ĒāĄė×ÓŅÖÖĘļ§øłĄė×ÓĖ®½ā£¬ĒŅĀĮĄė×ÓŅÖÖĘ³Ģ¶ČŠ”ÓŚĒāĄė×Ó£»
£Ø2£©¢ŁNH4Al£ØSO4£©2ĪŖĒæĖįČõ¼īŃĪ£¬ĘäČÜŅŗ³ŹĖįŠŌ£¬ÉżøßĪĀ¶Č“Ł½ųĖ®½ā£»
¢Śøł¾ŻČÜŅŗÖŠµÄµēŗÉŹŲŗć¼ĘĖć£»
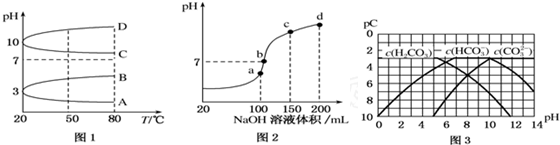
£Ø3£©a”¢b”¢c”¢dĖÄøöµć£¬øł¾Ż·“Ó¦ĮæµÄ¹ŲĻµ£¬aµćĒ”ŗĆĻūŗÄĶźH+£¬ČÜŅŗÖŠÖ»ÓŠ£ØNH4£©2SO4ÓėNa2SO4£»b”¢c”¢dČżµćČÜŅŗ¾łŗ¬ÓŠNH3•H2O£¬£ØNH4£©2SO4æÉŅŌ“Ł½ųĖ®µÄµēĄė£¬¶ųNH3•H2OŅÖÖĘĖ®µÄµēĄė£®bµćČÜŅŗ³ŹÖŠŠŌ£®
½ā“š ½ā£ŗ£Ø1£©NH4Al£ØSO4£©2ÓėNH4HSO4ÖŠµÄNH4+¾ł·¢ÉśĖ®½ā£¬µ«ŹĒNH4Al£ØSO4£©2ÖŠAl3+Ė®½ā³ŹĖįŠŌŅÖÖĘNH4+Ė®½ā£¬HSO4-µēĄė³öH+Ķ¬ŃłŅÖÖĘNH4+Ė®½ā£¬ŅņĪŖHSO4-µēĄėÉś³ÉµÄH+ÅØ¶Č±ČAl3+Ė®½āÉś³ÉµÄH+ÅØ¶Č“ó£¬ĖłŅŌNH4HSO4ÖŠNH4+Ė®½ā³Ģ¶Č±ČNH4Al£ØSO4£©2ÖŠµÄŠ”£¬
¹Ź“š°øĪŖ£ŗŠ”ÓŚ£»
£Ø2£©¢ŁNH4Al£ØSO4£©2ĪŖĒæĖįČõ¼īŃĪ£¬ĘäČÜŅŗ³ŹĖįŠŌ£¬ÉżøßĪĀ¶Č“Ł½ųĖ®½ā£¬µ¼ÖĀČÜŅŗĖįŠŌŌöĒ棬ČÜŅŗµÄpH¼õŠ”£¬ŌņĒśĻßA·ūŗĻ£¬
¹Ź“š°øĪŖ£ŗA£»
¢Śøł¾ŻµēŗÉŹŲŗćæÉµĆ£ŗ2c£ØSO42-£©-c£ØNH4+£©-3c£ØAl3+£©=c£ØH+£©-c£ØOH-£©=10-3 mol•L-1[c£ØOH-£©Ģ«Š”£¬æÉŗöĀŌ]£¬
¹Ź“š°øĪŖ£ŗ10-3£»
£Ø3£©a”¢b”¢c”¢dĖÄøöµć£¬øł¾Ż·“Ó¦ĮæµÄ¹ŲĻµ£¬aµćĒ”ŗĆĻūŗÄĶźH+£¬ČÜŅŗÖŠÖ»ÓŠ£ØNH4£©2SO4ÓėNa2SO4£»b”¢c”¢dČżµćČÜŅŗ¾łŗ¬ÓŠNH3•H2O£¬£ØNH4£©2SO4æÉŅŌ“Ł½ųĖ®µÄµēĄė£¬¶ųNH3•H2OŅÖÖĘĖ®µÄµēĄė£®bµćČÜŅŗ³ŹÖŠŠŌ£¬¼“ČÜŅŗŗ¬ÓŠ£ØNH4£©2SO4”¢Na2SO4”¢NH3•H2OČżÖֳɷ֣¬aµćŹ±c£ØNa+£©=c£ØSO42-£©£¬bµćŹ±c£ØNa+£©£¾c£ØSO42-£©£¬øł¾ŻNŌŖĖŲÓėSŌŖĖŲµÄ¹ŲĻµ£¬æÉŅŌµĆ³öc£ØSO42-£©£¾c£ØNH4+£©£¬¹Źc£ØNa+£©£¾c£ØSO42-£©£¾c£ØNH4+£©£¾c£ØOH-£©=c£ØH+£©£¬
¹Ź“š°øĪŖ£ŗa£»c£ØNa+£©£¾c£ØSO42-£©£¾c£ØNH4+£©£¾c£ØOH-£©=c£ØH+£©£®
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢ŃĪĄąĖ®½āµČÖŖŹ¶µć£¬ĢāÄæÄѶČÖŠµČ£¬Ąė×ÓÅØ¶Č“óŠ”±Č½Ļ³£³£ÓėŃĪĄąĖ®½ā”¢Čõµē½āÖŹµÄµēĄėĮŖŗĻæ¼²é£¬Č·¶ØĄė×ÓÅØ¶Č“óŠ”Ź±ŅŖ½įŗĻµēŗÉŹŲŗć”¢ĪļĮĻŹŲŗ楓·ÖĪö½ā“š£¬ÄѵćŹĒ£Ø3£©Ģā£¬ÖŖµĄĶ¼ĻóÖŠø÷øöµćµÄČÜÖŹ¼“æɽā“š£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H+”¢Na+”¢HCO${\;}_{3}^{-}$”¢Cl- | B£® | Mg2+”¢NO${\;}_{3}^{-}$”¢Na+”¢OH- | ||
| C£® | Ca2+”¢Cl-”¢CO${\;}_{3}^{2-}$”¢OH- | D£® | Na+”¢Cl-”¢K+”¢NO${\;}_{3}^{-}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗ¬16 gŃõŌ×ӵĶžŃõ»Æ¹č¾§ĢåÖŠŗ¬ÓŠµÄ¦Ä¼üŹżÄæĪŖ2NA | |
| B£® | 23.4 g NaCl¾§ĢåÖŠŗ¬ÓŠ0.1NAøöÓŅĶ¼ĖłŹ¾µÄ½į¹¹µ„ŌŖ | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬5 g D2Oŗ¬ÓŠµÄÖŹ×ÓŹż”¢µē×ÓŹż”¢ÖŠ×ÓŹż¾łĪŖ2.5NA | |
| D£® | 2 mol SO2ŗĶ1 mol O2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦ĖłµĆ»ģŗĻĘųĢå·Ö×ÓŹżŠ”ÓŚ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĻõĖįŅųČÜŅŗĒų·ÖĢ¼ĖįÄĘŗĶĀČ»ÆÄĘ | |
| B£® | ÓĆĻõĖį±µČÜŅŗĒų·ÖĢ¼ĖįÄĘŗĶĮņĖįÄĘ | |
| C£® | ÓĆĀČ»ÆøĘČÜŅŗĒų·ÖĢ¼ĖįÄĘŗĶĢ¼ĖįĒāÄĘ | |
| D£® | ÓĆŃĪĖįĒų·ÖĒāŃõ»ÆÄĘŗĶĀČ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1”Į10-11mol/L | B£® | 1”Į10-3mol/L | C£® | 1”Į10-7mol/L | D£® | 0.1mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻąĶ¬ĪĀ¶ČŹ±£¬Na2CO3 µÄČܽā¶ČŠ”ÓŚNaHCO3 µÄČܽā¶Č | |
| B£® | ³żČ„Ģ¼ĖįĒāÄĘ¹ĢĢåÖŠ»ģÓŠµÄÉŁĮæĢ¼ĖįÄĘæÉŅŌ²ÉÓĆ¼ÓČȵķ½·Ø | |
| C£® | ÓėĶ¬ÅضČĻ”ŃĪĖį·“Ó¦£¬NaHCO3·Å³öĘųÅŻµÄĖŁĀŹøüæģ | |
| D£® | ½«¶žÕßÅä³ÉČÜŅŗ£¬ŌŁ·Ö±šµĪ¼ÓCa£ØOH£©2ČÜŅŗ£¬ĪŽ°×É«³ĮµķÉś³ÉµÄŹĒNaHCO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1ÖÖ | B£® | 2ÖÖ | C£® | 3ÖÖ | D£® | 4ÖÖ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com