
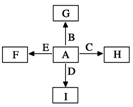
 ��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�����
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ����� �����Ŀռ乹����ֱ���ͷ��ӣ�
�����Ŀռ乹����ֱ���ͷ��ӣ����� A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�����
�ٷ�ӦC+G$\frac{\underline{\;����\;}}{\;}$B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ��÷�ӦΪ���ȷ�Ӧ����CΪAl��BΪFe��GΪFe2O3��HΪAl2O3��
��I��һ�ֳ������������壬��IΪCO2������E���Է�����Ӧ��2E+I$\frac{\underline{\;��ȼ\;}}{\;}$2F+D��F�е�EԪ�ص���������Ϊ60%����EΪMg��DΪC��FΪMgO����ͼ��ת����֪��AΪO2��Ȼ����Ԫ�ػ�����֪ʶ����ѧ���������
��� �⣺A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�����
�ٷ�ӦC+G$\frac{\underline{\;����\;}}{\;}$B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ��÷�ӦΪ���ȷ�Ӧ����CΪAl��BΪFe��GΪFe2O3��HΪAl2O3��
��I��һ�ֳ������������壬��IΪCO2������E���Է�����Ӧ��2E+I$\frac{\underline{\;��ȼ\;}}{\;}$2F+D��F�е�EԪ�ص���������Ϊ60%����EΪMg��DΪC��FΪMgO����ͼ��ת����֪��AΪO2��
��1�����з�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe���ʴ�Ϊ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe��
��2��IΪ������̼�������ʽΪ ��Ϊֱ���ͷ��ӣ��ʴ�Ϊ��
��Ϊֱ���ͷ��ӣ��ʴ�Ϊ�� ��ֱ���ͷ��ӣ�
��ֱ���ͷ��ӣ�
��3��8.0g G�����ʵ���Ϊ$\frac{8.0g}{160g/mol}$=0.05mol����Fe2O3��2Fe3+��Cu��֪����ҪCu������Ϊ0.05mol��64g/mol=3.2g��
�ʴ�Ϊ��3.2��
��4��C�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2������Ӧ����Һ������������Ӧ�Ļ�ѧ����ʽΪ��AlO2-+CO2+2H2O=HCO3-+Al��OH��3����CO2+OH-=HCO3-��
�ʴ�Ϊ��AlO2-+CO2+2H2O=HCO3-+Al��OH��3����CO2+OH-=HCO3-��
��5��E��I��ȼ�տ��ܹ۲쵽��������þ������ȼ�գ����ɰ�ɫ��ĩ����Ӧ���ڱڸ����к�ɫ��̼��
�ʴ�Ϊ��þ������ȼ�գ��ų�ҫ�۵İ⣬���ɰ�ɫ��ĩ����Ӧ���ڱڸ����к�ɫ��̼��
���� ���⿼��������ƶϣ�ע���������ȷ�Ӧ������ЧӦ���塢Mg�������̼�ķ�ӦΪ������ͻ�ƿڣ���ϤMg��Al���仯��������ʼ�������ԭ��Ӧ���ɽ����Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������������������й㷺��;��
��������������������й㷺��;���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ | B�� | ����Һ | C�� | ���� | D�� | ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢݢޢ� | B�� | �٢ڢܢݢ� | C�� | �ݢ� | D�� | �ܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢܢ� | C�� | �ڢ� | D�� | �ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ��һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����CO��CO2��O3���������������壬���Ƿֱ���1 mol��ԭ�ӣ���������������ʵ���֮��Ϊ�� ��
A��1��1��1 B��1��2��3 C��3��2��1 D��6��3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��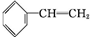 +Br2��
+Br2��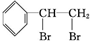 ��
��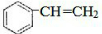 $\stackrel{����}{��}$
$\stackrel{����}{��}$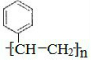 ��
��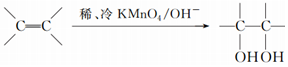 ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com