



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����֪�ڳ��³�ѹ�£�
��1����֪�ڳ��³�ѹ�£�| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
 �����۱�ϩ���Ƶ���Ľṹ��ʽ��
�����۱�ϩ���Ƶ���Ľṹ��ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ŀ�� | ʵ������O2 | ����ϡ������Һ | ���������Ƶ��۽��� | ���ε��ᴿ |
| װ�û���� |  | 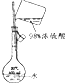 |  |  |
| ѡ�� | A | B | C | D |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ھ�����ֻҪ�������Ӿ�һ���������� |
| B����������������ˮ��ʱ����Һ��Ũ��һ������ |
| C��һ��ԭ�ӻ����ӣ��������8������ʱ��һ���ﵽ���ȶ��ṹ |
| D��ˮ�ķ�������������ƽ����������2/3�����ˮ���Ӽ�û��������������������ϾͲ�����Һ̬ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
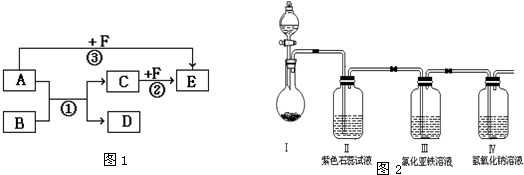
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

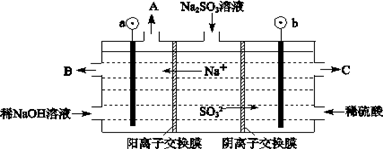
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ǵؿ��к������Ľ���Ԫ�� |
| B�����Ŀ���ʴ���ܺã�ԭ���������������Ĥ������ֹ��������е������Ӵ� |
| C������һ�ֻ��õĽ������ڻ�ѧ��Ӧ�г�����ԭ�� |
| D�����Ʋ;�����������ʱ���ż���ʳ��������˴������ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʹpH��ֽ�����ɫ����Һ�У�Fe3+��Cl-��Ba2+��Br-�ܴ������� | ||||
B�����Ե缫����Ȼ�����Һ��2Cl-+2H2O
| ||||
| C��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ��4Mg+6H++NO3-=4Mg2++NH4++3H2O | ||||
| D����10 mL 0.1 mol?L-1 KAl��SO4��2��Һ��10 mL 0.2 mol?L-1Ba��OH��2��Һ��ϣ��õ��ij�����Al��OH��3��BaSO4�����ʵ���֮��Ϊ1��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com