
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c(N2O3) |
| c(NO)?c(NO2) |
| c(N2O3) |
| c(NO)?c(NO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿���������ѧ�Ծ���A�������������� ���ͣ������
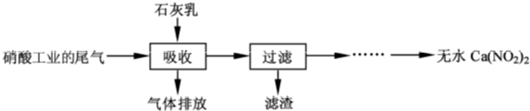
[2012�����վ�]��10�֣�����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��
��2�����������в�������Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����_____________ _________��������ѭ��ʹ�ã���������Ҫ�ɷ���__________(�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO)��n(NO2)��1��1����ᵼ��______________________ ___����n(NO)��n(NO2)��1��1����ᵼ��_________________________________��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ�����߿�ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
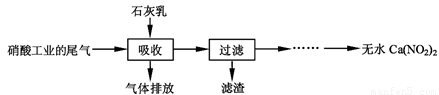
����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
��ش��������⣺
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK=
��
N2O3(g)����ƽ�ⳣ������ʽΪK=
��
��2�����������в�����Һ�����Ӵ�����(β�����������ײ����룬ʯ�������������������)����Ŀ���� ��������ѭ�����ã���������Ҫ�ɷ��� (�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1�U1����n(NO) ��n(NO2)��1�U1,��ᵼ�� ����n(NO) ��n(NO2)��1�U1,��ᵼ�� ��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���¿α���������¿���������ѧ�Ծ���A�����������棩 ���ͣ������
[2012�����վ�]��10�֣�����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�

��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��
��2�����������в�������Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����_____________ _________��������ѭ��ʹ�ã���������Ҫ�ɷ���__________(�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO)��n(NO2)��1��1����ᵼ��______________________ ___����n(NO)��n(NO2)��1��1����ᵼ��_________________________________��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com