

������1����ɲ���ƽ���з�Ӧ����ʽ��
������ ��![]() ���� ��HCl������
��
���� ��HCl������
��![]() ���� ������ ������ ��
���� ������ ������ ��
������2��Ҫ�õ�������X���壬AƿӦʢ________��BƿӦʢ________��
������3����C���İ�ɫ������________��Dƿ���Ϻ�ɫ�ľ�����________����C�����ȵ�Ŀ����________��
������4��E��Ӧ����________װ�ã�ԭ����_______________________________��
������5����λͬѧ��ѭ�����ô�Dƿ������X���壬Ϊ������Dƿ����һ������F��Bƿ������Ϊ���𣿣�˵��ԭ��
����______________________________________________________��
| ��1�� ��2������ʳ��ˮ��ŨH2SO4����3��NaI��KI��I2��ʹI2��������Dƿ����4��ʢ��NaOH��Һ��β������װ�ã�����д�
|
| ��1��
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 Ϊ�ⶨNa3CO3��Na2SO3������и���ֵĺ�����ȡ��Ʒ23.2g������ͼ��ʾװ�ý���ʵ�飨����̨�����е�����δ��ͼ�л�������
Ϊ�ⶨNa3CO3��Na2SO3������и���ֵĺ�����ȡ��Ʒ23.2g������ͼ��ʾװ�ý���ʵ�飨����̨�����е�����δ��ͼ�л��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ��һ��B����Һ�������� | �ڶ���B����Һ�������� | |
| �� | ��Fe2+����Fe3+ | ��SO 42- |
| �� | ����Fe3+������Fe2+ | ��SO 42- |
| �� | ��Fe3+����Fe2+ | ��Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
����ͼ��ʾװ���е�Ũ�������ظ����(![]() )����ʱ��������ɫ��̬����X����A��B��ƿ����C�����ȵ�ij��ɫ���巴Ӧ�����Dƿ���Ϻ�ɫ��״����֣�
)����ʱ��������ɫ��̬����X����A��B��ƿ����C�����ȵ�ij��ɫ���巴Ӧ�����Dƿ���Ϻ�ɫ��״����֣�
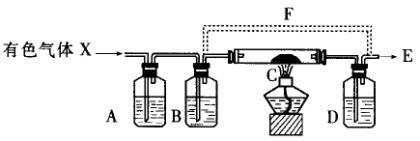
������1����ɲ���ƽ���з�Ӧ����ʽ��
������ ��![]() ���� ��HCl������
��
���� ��HCl������
��![]() ���� ������ ������ ��
���� ������ ������ ��
������2��Ҫ�õ�������X���壬AƿӦʢ________��BƿӦʢ________��
������3����C���İ�ɫ������________��Dƿ���Ϻ�ɫ�ľ�����________����C�����ȵ�Ŀ����________��
������4��E��Ӧ����________װ�ã�ԭ����_______________________________��
������5����λͬѧ��ѭ�����ô�Dƿ������X���壬Ϊ������Dƿ����һ������F��Bƿ������Ϊ���𣿣�˵��ԭ��
����______________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com