
��ҵ�������е�һ����Ҫ��Ӧ��SO2��400��500���µĴ�������2SO2 + O2 2SO3������һ������Ӧ���ȵĿ��淴Ӧ�������Ӧ���ܱ������н��У������й�˵���д������
2SO3������һ������Ӧ���ȵĿ��淴Ӧ�������Ӧ���ܱ������н��У������й�˵���д������
A��ʹ�ô�����Ϊ�˼ӿ췴Ӧ���ʣ��������Ч��
B�������������£�SO2������100����ת��ΪSO3
C��Ϊ�����SO2��ת���ʣ�Ӧ�ʵ����O2��Ũ��
D���ﵽƽ��ʱ��SO2��Ũ����SO3��Ũ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������(����)
A��SO2ʹ��ˮ��ɫ����ϩʹKMnO4��Һ��ɫ��ԭ����ͬ
B���Ʊ���������ʱ�����ȵ�NaOH��Һ�ռ������Գ�ȥ���е�����
C���ñ���ʳ��ˮ���ˮ����ʯ��Ӧ�����Լ�����Ȳ�IJ�������
D����AgNO3��Һ���Լ���KCl��KI
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڶ����ԭ���У������������������Щ���ؾ���
�ٵ��Ӳ��ԭ�ӹ�����ͣ������Dz㣩�ۿռ���չ���������״̬
A���٢ڡ���B���٢ܡ���C���ڢۡ���D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӻ��ȡ������ܡ����ܵĵ�λ����kJ��mol-1
| ���� | ����ԭ�ӻ��� | ���ӻ����� | ������ | ���ۼ� | ���� |
| Na | 108.4 | NaCl | 786 | Cl-Cl | 243 |
| Mg | 146.4 | NaBr | 747 | Si-Si | 176 |
| Al | 326.4 | MgO | 3791 | Si-Cl | 360 |
������˵����ȷ����
A��Na(s)��Cl2��g����Ӧ����1molNaCl(s)�ų�������Ϊ556.1kJ
B��Si(s)+2Cl2(g)=SiCl4(g) ��H= �� 602kJ��mol-1
C���ӱ��п��Կ������Ȼ��Ƶ��۵�Ⱦ�����
D���ӱ������ݿ��Կ��������뾶Խ������������Ӽ���Խ���������ۼ�ȴԽǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͬλ����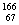 Ho��ԭ�Ӻ��ڵ�����������������֮����
Ho��ԭ�Ӻ��ڵ�����������������֮����
A��32 B��67 C��99 D��166
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������γ���Ҫ������
A��ɭ����ҿ��ķ����ƻ�����̬���� B������ʯȼ�ϵĴ���ȼ��
C�������ж�����̼�ĺ������� D�������ŷŴ���β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ���ձ��ж�ʢ��ϡ���ᡣ
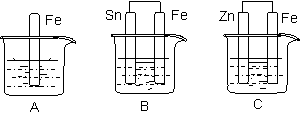 |
��1��A�з�Ӧ�����ӷ���ʽΪ ��
��2��B�еĵ缫��Ӧ��Fe�� ��Sn�� ��Sn��������Һ��pH��������С�䣩 ��
��3��C�б���ʴ�Ľ����� ����缫��ӦʽΪ ��
�Ƚ�A��B��C�д�������ʴ�������ɿ쵽����˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
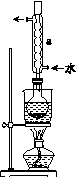
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s) Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O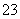 ��I2===S4O
��I2===S4O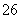 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
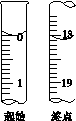
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO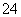 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鷽���У����ܴﵽʵ��Ŀ�ĵ���(����)
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ����CH3CH2Br��NaOH��Һ���Ƿ���ˮ�� | ��CH3CH2Br��NaOH��Һ���ȡ���ȴ��ȡ���ϲ�ˮ��Һ����ϡHNO3�ữ������AgNO3��Һ���۲��Ƿ��������ɫ���� |
| B | ����Fe(NO3)2�����Ƿ����������� | ��Fe(NO3)2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
| C | ��֤Br2��������ǿ��I2 | ��������ˮ����KI��Һ�У��ټ���CCl4�������ã��ɹ۲쵽�²�Һ�����ɫ |
| D | ��֤Fe(OH)3���ܽ��С��Mg(OH)2 | ��FeCl3��Һ����Mg(OH)2����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ���ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com