
 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ϩ�Ľṹ��ʽCH2CH2 | B������ķ���ʽC2H4O2 |
| C�������Ļ�ѧʽKAlSO4��12H2O | D���Ȼ��Ƶĵ���ʽ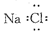 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��������̼�ĵ���ʽΪ�� | B����ϩ�Ľṹ��ʽΪ��CH2CH2 |
| C���Ҵ��ķ���ʽΪ��C2H6O | D��Cl�����ӵĽṹʾ��ͼΪ��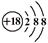 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2Na + 2H2O �� 2NaOH + H2�� | B�� Na2O + H2O �� 2NaOH |
C�� H2O  H2��+ O2�� H2��+ O2�� | D��C + H2O CO + H2 CO + H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������ķ��ӱ���ģ��ͼ�� | B���۱�ϩ�ṹ��ʽ��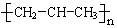 |
| C��ȩ���Ľṹ��ʽ����COH | D����ϩ�Ľṹ��ʽ��CH2CH2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ�����ɹ��������Ƶ�1mol����ת�Ƶĵ�������2NA |
| B�������22��4L�ı���ȫȼ�����ɵ�CO2�������134��4L |
| C��1L0��1mol��L��1����ˮ�к��еķ�����С��0��1NA |
| D��1L0��5mol��L��1��Na2CO3��Һ�к��е�������Ŀ����1��5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
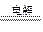 MnO2 + Zn + 2H2SO4
MnO2 + Zn + 2H2SO4| A������MnO2��H2SO4���������� | B����������16 g Sʱת��1 mol ���� |
| C������MnSO4����������Ӧ | D�������ڸ������п�ѭ��ʹ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com