
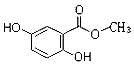
 ��
��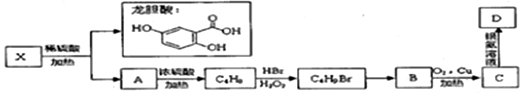
 ��C4H9Br��B�Ļ�ѧ��Ӧ��ȡ����Ӧ��
��C4H9Br��B�Ļ�ѧ��Ӧ��ȡ����Ӧ�� ��
������ ��1��������������Ľṹ��ʽ�жϺ��еĹ����ţ�
��2�������к��з��ǻ������зӵ����ʣ����ǻ�������С��̼�ᣬ��̼���Ʒ�Ӧ����̼�����ƣ����������������������ʣ��ݴ˽��ѡ����
��3�����ǻ������������������Ʒ�����Ӧ��
��X��ϡ���ᡢ����������������������A��Ϊ����ˮ�ⷴӦ��AΪ����A������ȥ��Ӧ����C4H8��A�к�����������AΪ��CH3��3COH��C4H8Ϊ��CH3��2C=CH2����HBr�����ӳɷ�Ӧ����C4H9Br��C4H9Br����±������ˮ�ⷴӦ����B��B������������������Ӧ����C4H9BrΪ��CH3��2CHCH2Br��BΪ��CH3��2CH2CH2OH��CΪCΪ��CH3��2CHCHO��DΪ��CH3��2CHCOOH����XΪ ���ݴ˽��
���ݴ˽��
��� �⣺��1��������������Ľṹ��ʽ����֪���еĹ������У��������ǻ����ʴ�Ϊ���������ǻ���
��2��A�����ܷ�����ȥ��Ӧ����A��ȷ��
B�����з��ǻ���������ˮ�����ڶ�λȡ����Ӧ������1mol����������������3mol�巴Ӧ����B��ȷ��
C�����������������������ʣ�������ˮ����C��ȷ��
D���ӵ����Ա�̼���������ǻ�����������̼������Һ��Ӧ����������̼����D����
�ʴ�Ϊ��D��
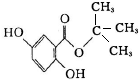
��3���������������������������Һ����ˮ�ⷴӦ��ͬʱ���������������Ƶ��кͷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4���������Ϸ�����X�Ľṹ��ʽΪ ��C4H9Br��B ��Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCH2Br+NaOH$��_{��}^{H_{2}O}$��CH3��2CHCH2OH+NaBr������ȡ����Ӧ���ʴ�Ϊ��
��C4H9Br��B ��Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCH2Br+NaOH$��_{��}^{H_{2}O}$��CH3��2CHCH2OH+NaBr������ȡ����Ӧ���ʴ�Ϊ�� ��ȡ����
��ȡ����
��5��C��D ��Ӧ�Ļ�ѧ����ʽΪ����CH3��2CHCHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$��CH3��2CHCOONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ����CH3��2CHCHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$��CH3��2CHCOONH4+2Ag��+3NH3+H2O��
��6�����������������������һ��ͬ���칹��Ľṹ��ʽ�����ܷ���������Ӧ������ȩ�� ����ʹFeCl3��Һ��ɫ�����з��ǻ��������࣬��Ϣٿ�֪��ӦΪ�����γɵ��������ܺ˴Ź������ķ�ֵΪ1��1��2��2�������������ͬ���칹��Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϡ������ŵ����ʡ�ͬ���칹��ȣ��Ѷ��еȣ�ע�����չ����ŵ�������ת����


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ժ�ɫ����Һ�У�Al3+��NH4+��SO42-��MnO4- | |
| B�� | ������KMnO4��Һ�У�SO42-��Mg2+��NO3-��CH3CH2OH | |
| C�� | ��������ˮ�������c��H+��•c��OH-��=10-20 mol2•L-2����Һ�У�Na+��NH4+��Cl-��SiO32- | |
| D�� | ʹpH��ֽ��������Һ�У�NH4+��Na+��SO42-��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������Һ�백ˮ��ϣ�CH3COOH+NH3•H2O�TCH3COO-+NH4++H2O | |
| B�� | ͭƬ������������Һ�У�Cu+Ag+�TCu2++Ag | |
| C�� | ������C12��NaOH��Һ��Ӧ��Cl2+2OH-�TC1-+C1O-+H2O | |
| D�� | ����������Һ�м���ϡ���Ba2++SO42-�TBaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl��H2SO4��S | B�� | ���ʯ��Na3PO4��Mg | ||
| C�� | HF��SiC��Ar | D�� | H2O��SiO2��K2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ���������м��Ӧ��3Fe+8H++2NO3-=3Fe3++2NO��+4H2O�� | |
| B�� | ��������Һ�����İ�ˮ��Ӧ��Ag++3NH3•H2O�TAg��NH3��2OH+NH4++2H2O | |
| C�� | ��NH4Al��SO4��2��Һ�м��������Ba��OH��2��Һ��Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O | |
| D�� | ̼������Һ�м���������ӣ� +CO32-�� +CO32-�� +HCO3- +HCO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com