
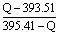
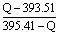 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1��1 | B��1��3 | C��1��4 | D��2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��H2O��g�� ��H1��a kJ��
��H2O��g�� ��H1��a kJ��
 ��2H2O��g�� ��H2��b kJ��
��2H2O��g�� ��H2��b kJ��
 ��H2O��l�� ��H3��c kJ��
��H2O��l�� ��H3��c kJ��
 ��2H2O��l�� ��H4��d kJ��
��2H2O��l�� ��H4��d kJ��
| A��a��c ��0 | B��b��d��0 | C��2a��b��0 | D��2c��d��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A����NA�ĸ�����ת��ʱ���÷�Ӧ�ų�1300KJ������ |
| B����NA��ˮ����������ΪҺ��ʱ������1300KJ������ |
| C����2NA��̼�����õ��Ӷ�����ʱ���ų�1300KJ������ |
| D����8NA��̼�����õ�������ʱ���ų�1300KJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
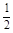 O2(g) ��H2O(l) ��H2���C285.8 kJ/mol
O2(g) ��H2O(l) ��H2���C285.8 kJ/mol| A����H��244.1kJ/mol | B����H��-488.3kJ/mol |
| C����H��-996.6kJ/mol | D����H��996.6kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�� �� ��
2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�� �� ��
A��N2(g)+3H2(g)  2NH3(1)����H=2(a-b-c)kJ��mol-1 2NH3(1)����H=2(a-b-c)kJ��mol-1 |
B��N2(g)+3H2(g)  2NH3(g)����H=-2(b-a)kJ��mol-1 2NH3(g)����H=-2(b-a)kJ��mol-1 |
C�� N2(g)+ N2(g)+ H2(g) H2(g)  NH3(1)����H=(b+c-a)kJ��mol-1 NH3(1)����H=(b+c-a)kJ��mol-1 |
D�� N2(g)+ N2(g)+ H2(g) H2(g)  NH3(g)����H=(a+b)kJ��mol-1 NH3(g)����H=(a+b)kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
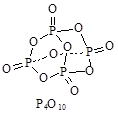
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���к��Ȧ�H����57.3 kJ��mol��1,, ��2H��(aq)�� (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 |
| B����0.5 molN2��1.5 mol H2���ܱ���������NH3(g)������19.3 kJ������ʽΪ�� N2(g)+3H2(g)  2NH3(g)��H="��38.6" kJ��mol��1 2NH3(g)��H="��38.6" kJ��mol��1 |
| C����״����H2(g)��F2(g) ==="2HF(g)" ��H����270kJ/mol�� |
| D����������4NH3(g)��5O2(g) ===4NO(g)��6H2O(g)��H����1025 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���� | B���ڢۢ� | C���� | D���٢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com