
ĻĀĶ¼ÖŠ£¬A”¢CŹĒ¹¤ŅµÉĻÓĆĶ¾ŗܹćµÄĮ½ÖÖÖŲŅŖ»Æ¹¤ŌĮĻ£¬BĪŖČÕ³£Éś»īÖŠ³£¼ūµÄ½šŹō£¬
![]() H”¢GŹĒÕżĖÄĆęĢå½į¹¹µÄ·Ē¼«ŠŌ·Ö×Ó£¬HŹĒŅ»ÖÖÖŲŅŖµÄÄÜŌ“£¬JŹĒŅ»ÖÖÄĶøßĪĀ²ÄĮĻ£¬KŹĒÓÉ
H”¢GŹĒÕżĖÄĆęĢå½į¹¹µÄ·Ē¼«ŠŌ·Ö×Ó£¬HŹĒŅ»ÖÖÖŲŅŖµÄÄÜŌ“£¬JŹĒŅ»ÖÖÄĶøßĪĀ²ÄĮĻ£¬KŹĒÓÉ
![]() Į½ÖÖ³£¼ūŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ£ØĶ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļƻӊĮŠ³ö£©”£Ēė°“ŅŖĒó»Ų“š£ŗ
Į½ÖÖ³£¼ūŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ£ØĶ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļƻӊĮŠ³ö£©”£Ēė°“ŅŖĒó»Ų“š£ŗ
![]()
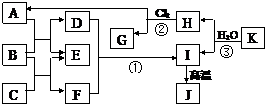 (1)Š“³öBµÄ»ÆѧŹ½______________£¬
(1)Š“³öBµÄ»ÆѧŹ½______________£¬
![]() GµÄµē×ÓŹ½ ”£
GµÄµē×ÓŹ½ ”£
![]() (2)·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ_________________________________________________
(2)·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ_________________________________________________
![]() (3)·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ________________________________________________
(3)·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ________________________________________________
(4)·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ ______________________________________________
(1)Al ”¤”¤ ”¤”¤ (2)Al3++3-2+6H2O==== 4Al(OH)3”ż
![]() (3)¹āÕÕ ¹żĮæCl2 (4)Al4C3+12H2O=== 4Al(OH)3+3CH4”ü
(3)¹āÕÕ ¹żĮæCl2 (4)Al4C3+12H2O=== 4Al(OH)3+3CH4”ü
±¾ĢāĪŖæņĶ¼ĶʶĻĢā£¬Ö÷ŅŖæ¼²éĀĮ¼°Ęä»ÆŗĻĪļµÄŠŌÖŹÓėÖŲŅŖÓ¦ÓĆ”£ÓÉH”¢GŹĒÕżĖÄĆęĢå
![]() ½į¹¹µÄ·Ē¼«ŠŌ·Ö×Ó£¬ĒŅH+Cl2”śA+GÖŖHĪŖCH4£¬GĪŖCCl4,AĪŖHCl;ĮŖĻėŅŅČ²µÄÖĘ·Ø¼°K
½į¹¹µÄ·Ē¼«ŠŌ·Ö×Ó£¬ĒŅH+Cl2”śA+GÖŖHĪŖCH4£¬GĪŖCCl4,AĪŖHCl;ĮŖĻėŅŅČ²µÄÖĘ·Ø¼°K
![]() ÓÉĮ½ÖÖ³£¼ūŌŖĖŲ×é³É£¬æÉĶĘ²āIĪŖ½šŹōĒāŃõ»ÆĪļ£¬ÓÖIøßĪĀJ(øßĪĀ²ÄĮĻ)£¬ŌņĘäÖŠ½šŹōæÉÄÜĪŖ
ÓÉĮ½ÖÖ³£¼ūŌŖĖŲ×é³É£¬æÉĶĘ²āIĪŖ½šŹōĒāŃõ»ÆĪļ£¬ÓÖIøßĪĀJ(øßĪĀ²ÄĮĻ)£¬ŌņĘäÖŠ½šŹōæÉÄÜĪŖ
![]() Mg»ņAl”£ÓÖHCl+B”śD+E£¬B+C”śF+E£¬D+F”śI(½šŹōĒāŃõ»ÆĪļ)£¬æÉÖŖEĪŖH2£¬IĪŖAl(OH)3£¬
Mg»ņAl”£ÓÖHCl+B”śD+E£¬B+C”śF+E£¬D+F”śI(½šŹōĒāŃõ»ÆĪļ)£¬æÉÖŖEĪŖH2£¬IĪŖAl(OH)3£¬
![]() BĪŖAl,CĪŖNaOH£¬Č«²æĪļÖŹÖĮ“ĖČ«²æČ·¶Ø”£
BĪŖAl,CĪŖNaOH£¬Č«²æĪļÖŹÖĮ“ĖČ«²æČ·¶Ø”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

Ēė°“ŅŖĒó»Ų“š£ŗ
£Ø1£©Š“³öBµÄ»ÆѧŹ½___________£¬GµÄµē×ÓŹ½___________”£
£Ø2£©·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________________”£
£Ø3£©·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ___________£¬___________”£
£Ø4£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

Ēė°“ŅŖĒó»Ų“š£ŗ
£Ø1£©Š“³öBµÄ»ÆѧŹ½______________£¬GµÄµē×ÓŹ½______________________”£
£Ø2£©·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ________________________________________”£
£Ø3£©·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ__________________________£¬________________________”£
£Ø4£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

Ēė°“ŅŖĒóĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öBµÄ»ÆѧŹ½_____________________£¬GµÄµē×ÓŹ½______________________”£
£Ø2£©·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ______________________________________”£
£Ø3£©·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ________________________________________”£
£Ø4£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

Ēė°“ŅŖĒóĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öBµÄ»ÆѧŹ½______________£¬GµÄµē×ÓŹ½______________”£
£Ø2£©·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________________”£
£Ø3£©·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ______________”¢______________”£
£Ø4£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼ÖŠ£¬A”¢CŹĒ¹¤ŅµÉĻÓĆĶ¾ŗܹćµÄĮ½ÖÖÖŲŅŖ»Æ¹¤ŌĮĻ£¬BĪŖČÕ³£Éś»īÖŠ³£¼ūµÄ½šŹō£¬H”¢GŹĒÕżĖÄĆęĢå½į¹¹µÄ·Ē¼«ŠŌ·Ö×Ó£¬HŹĒŅ»ÖÖÖŲŅŖµÄÄÜŌ“£¬JŹĒŅ»ÖÖÄĶøßĪĀ²ÄĮĻ£¬KŹĒÓÉĮ½ÖÖ³£¼ūŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ£ØĶ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļƻӊĮŠ³ö£©”£

Ēė°“ŅŖĒó»Ų“š£ŗ
£Ø1£©Š“³öBµÄ»ÆѧŹ½___________£¬GµÄµē×ÓŹ½___________”£
£Ø2£©·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________________”£
£Ø3£©·“Ó¦¢Ś½ųŠŠµÄĢõ¼žŹĒ___________£¬___________”£
£Ø4£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ĪŖ____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com