
������������⣺?
��1�����������γ�����������Ĥ�ĵ缫��Ӧʽ�����ӷ�Ӧʽ�ֱ�Ϊ�������������������� ��������
��2���������У�����ʹ���ҺpH��������ȶ�������̫��Ҳ����̫С����ԭ������������������?
��3��ʹ��NaHCO3��ҺΪ���Һ���������������ҺpH��������˵����һԭ�������ӷ���ʽΪ����������������
(1)Al-3e-![]() Al3+,Al3++3HCO
Al3+,Al3++3HCO![]()
![]() Al(OH)3��+3CO2��?
Al(OH)3��+3CO2��?
(2)��ΪAl2O3��Al(OH)3�����������ʣ����Һ��pH̫���̫С����ʹ���ܽ�?
��3��OH-+HCO![]()
![]() CO
CO![]() +H2O?
+H2O?
������(1)���ʱ��������ӦΪAl-3e-![]() Al3+��������������Al3+��HCO
Al3+��������������Al3+��HCO![]() ����ˮ������ٽ���Al3++3HCO
����ˮ������ٽ���Al3++3HCO![]()
![]() Al(OH)3��+3CO2����
Al(OH)3��+3CO2����
(2)��ΪAl2O3��Al(OH)3��Ϊ�������ʣ�pH̫���̫С����ʹAl2O3��Al(OH)3�ܽ⡣?
��3����Ϊ����������Ӧ��2H+-2e-![]() H2��,OH-������OH-������������HCO
H2��,OH-������OH-������������HCO![]() ��Ӧ���Ӷ�������������ҺpH������
��Ӧ���Ӷ�������������ҺpH������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2004ȫ����ʡ�и߿�ģ�������ࡤ��ѧ ���ͣ�022
����������ɫ����Ӧ�õ�ⷨʹ�����渽��һ�����������䷽���ǽ�������������������������̼�����������Һ�����е�⣮��ԭ���ǣ�ͨ�������������Һ�ĽӴ��������γ�һ��Al(OH)3��Ĥ����Ĥ��ijЩ��λ������С�ף�������С��ͨ������������ʹAl(OH)3�ֽ⣬�Ӷ������������γ�һ��Ϻ������Ĥ��
�Իش�
(1)���������γ�����������Ĥ�ĵ缫��Ӧʽ�����ӷ�ӦʽΪ��________��
(2)�������У�����ʹ���ҺpH��������ȶ�(����̫��Ҳ����̫С)��ԭ����________��
(3)ʹ��NaHCO3��ҺΪ���Һ���������������ҺpH��������˵����һԭ�������ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
�����Իش�
������1�����������γ�����������Ĥ�ĵ缫��Ӧʽ�����ӷ�ӦʽΪ��________.
������2���������У�����ʹ���ҺpH��������ȶ�������̫��Ҳ����̫С����ԭ����________.
������3��ʹ��![]() ��ҺΪ���Һ���������������ҺpH��������˵����һԭ�������ӷ���ʽΪ________________.
��ҺΪ���Һ���������������ҺpH��������˵����һԭ�������ӷ���ʽΪ________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼʾ����һ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
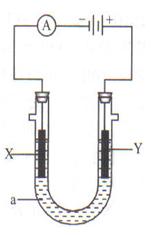
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��Ӧʽ�ǣ�
______________________________________________________
��X�������۲쵽�������ǣ�_____________________________
______________________________________________________
��Y�缫�ϵĵ缫��Ӧʽ�ǣ�_____________________________
����õ缫��Ӧ����ķ����ǣ�___________________________
_____________________________________________________��
�ܷ�Ӧ��ѧ����ʽ�ǣ�_____________________________________��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ�����_________����Y�缫�IJ�����_________��
��3����Ҫ�����������ͭ������Һaѡ��_______________��X�缫�IJ�����____________��Y�缫�IJ�����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com