
 ij»ÆѧŠĖȤŠ”×éÖĘČ”²¢½ųŠŠŅŅĻ©ŠŌÖŹµÄĢ½¾æŹµŃ飬ÖʵƵÄŅŅĻ©ÖŠæÉÄÜŗ¬ÓŠSO2”¢CO2µČĘųĢ壬½«ÖʵƵÄŅŅĻ©ĶعżäåĖ®Ź±£¬æɹŪ²ģµ½ŹŌ¹ÜÖŠäåĖ®ĶŹÉ«£®
ij»ÆѧŠĖȤŠ”×éÖĘČ”²¢½ųŠŠŅŅĻ©ŠŌÖŹµÄĢ½¾æŹµŃ飬ÖʵƵÄŅŅĻ©ÖŠæÉÄÜŗ¬ÓŠSO2”¢CO2µČĘųĢ壬½«ÖʵƵÄŅŅĻ©ĶعżäåĖ®Ź±£¬æɹŪ²ģµ½ŹŌ¹ÜÖŠäåĖ®ĶŹÉ«£®


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| O | - 2 |
| O | - 2 |
| O | 2- 6 |
| 90.5cV |
| 4m |
| 90.5cV |
| 4m |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | |
| ²½Öč1 | ŌŚŹŌ¹ÜÖŠ¼ÓČė¹żĮæµÄæéדĢ¼ĖįøĘ£¬ŌŁ¼ÓČėŌ¼20mL±„ŗĶĀČĖ®£¬³ä·Ö·“Ó¦£¬¹żĀĖ£¬½«ĀĖŅŗ·ÖĪŖČōøÉ·Ż | ÓŠÉŁĮæĘųÅŻ²śÉś£¬ČÜŅŗµÄ»ĘĀĢÉ«ĶŹČ„ |
| ²½Öč2 | ½«µŚŅ»·ŻĀĖŅŗÓėĻ”ŃĪĖį»ģŗĻ | ²śÉś“óĮæĘųÅŻ |
| ²½Öč3 | ½«µŚ¶ž·ŻĀĖŅŗ¼ÓČČ | ČÜŅŗ±ä»ė×Ē£¬ĒŅÓŠ“óĮæĪŽÉ«ĘųĢå²śÉś |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
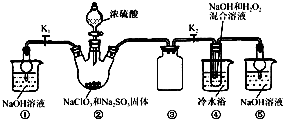
 ij»ÆѧŠĖȤŠ”×éÖĘČ”²¢½ųŠŠŅŅĻ©ŠŌÖŹµÄĢ½¾æŹµŃ飬ÖʵƵÄŅŅĻ©ÖŠæÉÄÜŗ¬ÓŠSO2”¢CO2µČĘųĢ壬½«ÖʵƵÄŅŅĻ©ĶعżäåĖ®Ź±£¬æɹŪ²ģµ½ŹŌ¹ÜÖŠäåĖ®ĶŹÉ«£®
ij»ÆѧŠĖȤŠ”×éÖĘČ”²¢½ųŠŠŅŅĻ©ŠŌÖŹµÄĢ½¾æŹµŃ飬ÖʵƵÄŅŅĻ©ÖŠæÉÄÜŗ¬ÓŠSO2”¢CO2µČĘųĢ壬½«ÖʵƵÄŅŅĻ©ĶعżäåĖ®Ź±£¬æɹŪ²ģµ½ŹŌ¹ÜÖŠäåĖ®ĶŹÉ«£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011Ń§ÄźÉ½¶«Ź”ĮŁŅŹŹŠ²ŌɽŅ»ÖŠøßŅ»£ØĻĀ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com