
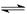 CH3COO-+H+
CH3COO-+H+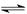 Fe(OH)3+3H+ ����Fe3+ˮ��
Fe(OH)3+3H+ ����Fe3+ˮ��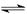 CH3COO-+H+��
CH3COO-+H+��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼��һ����̼ | B��1moL̼��2moL̼ |
| C��1moL��Ȳ��2moL̼ | D�����ۺ���ά�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���� �� �� �� �� | B����=�� �� �� |
C���� �� �� �� �� | D����=��=�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��PH=7����Һ | B��c(H+)=10-7mol/L����Һ |
| C�����ǡ����ȫ�к͵���Һ | D��c(H+)= c(OH��)=3.5��10-7mol/L����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
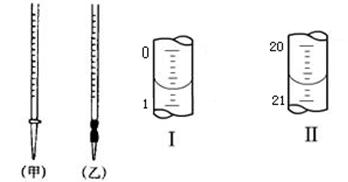
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.1 mol/L | B��1��10-11 mol/L | C��1��10-7 mol/L | D��1��10-3 mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij������Һ��pH��a��������Һϡ��10������Һ��pH��b����a>b |
| B�������£�ij��Һ����ˮ�����c(OH-) = 1.0��10-13�������Һһ�������� |
| C��25��ʱ����pH��4������ϡ��1000������Һ��pH��7 |
| D��25��ʱ��pH=13��ǿ����Һ��pH=2��ǿ����Һ��ϣ������û��Һ��pH=7����ǿ����ǿ����������1:10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����H1����H2����H3 | B����H1����H2����H3 |
| C����H1����H2����H3 | D����H1����H2����H3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����28.7 kJ/mol |
| B��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����28.7 kJ/mol |
| C��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.4 kJ/mol |
| D��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.4 kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com