
���������ʾ��ij���е���Ҫ������Ⱦ��ΪSO2��NOx��CO�ȣ�����Ҫ��ԴΪȼú��������β�������������о���
��1��Ϊ����ȼú��SO2���ŷţ��ɽ�úת��Ϊ���ȼ��ˮú����CO��H2����
��֪��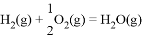 ��H��241.8kJ��mol��1��
��H��241.8kJ��mol��1��
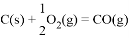 ��H����110.5kJmol��1
��H����110.5kJmol��1
д����̿��1molˮ������Ӧ����ˮú�����Ȼ�ѧ����ʽ��________��
��2������β����NO���ڷ��������������ɵģ���ӦΪN2��g����O2��g�� 2NO��g�� ��H��0��
2NO��g�� ��H��0��
�ٽ���0.8molN2��0.2molO2�����ƿ�����ɣ��Ļ���������ij�ܱ������У�����1300�淴Ӧ�ﵽƽ�⣬�������8��10��4molNO��������¶��´˷�Ӧ�Ļ�ѧƽ�ⳣ��K��________������Ƽ���������
�������������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ����________��
��3��������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�������������ų�����Һ����NO2��
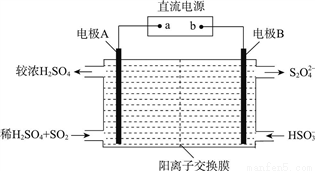
�ٵ缫A�ĵ缫��ӦʽΪ________��
�缫B�ĵ缫��ӦʽΪ________��
�ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ����SO32�����÷�Ӧ�����ӷ���ʽΪ________��
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
���������к��й��ۼ������ӻ�������
A. CaCl2 B. NH4HCO3 C. Na2O D. H2O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ܱ������A��B��Ӧ����C���䷴Ӧ���ʷֱ���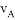 ��
��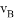 ��
��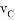 ��ʾ����֪2
��ʾ����֪2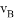 =3
=3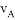 ��3
��3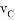 =2
=2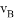 ����˷�Ӧ�ɱ�ʾΪ
����˷�Ӧ�ɱ�ʾΪ
A. 2A+3B=2C B. A+3B=2C
C. 3A+B=2C D. A+B=C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������������ԭ�����͵���
A. �����ڳ�ʪ�Ŀ�������������
B. �غ�ɫNO2��ѹ����ɫ�ȱ�����dz
C. ���¼������������ʹ�ϳɰ��ķ�Ӧ���ʼӿ�
D. H2��I2��HIƽ��������ѹ����ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͨ�����ǰѲ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ��ָ�����ѧ���ļ������£�
��ѧ�� | H��H | Cl��Cl | Cl��H |
����/(kJ��mol��1) | 436 | 243 | 431 |
�����H2(g)��Cl2(g) === 2HCl(g)�ķ�Ӧ��
A. ��862 kJ��mol��1 B. ��679 kJ��mol��1 C. ��183 kJ��mol��1 D. ��183 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�����£�0.1mol��L��1��NaHSO3��Һ��pH��4������Һ�и����ӵ�Ũ�ȹ�ϵ��ȷ����
A. C��Na������c��HSO3������C��H������c��OH����
B. c��H2SO3����c��SO32����
C. c��H������C��Na������c��OH������c��SO32������c��HSO3����
D. c��Na������C��HSO3������c��H2SO3����2c��SO32����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A. ��ֱ�Ӽ��Ȳ�����FeCl3��Һ�ķ������Ƶô�������ˮFeCl3
B. �����ʵ���Ũ�ȵ�Na2CO3��Һ��CH3COONa��Һ��pH��ǰ�ߴ��ں���
C. �к͵�����������ʵ���Ũ�ȵ�����ʹ�����Һ������NaOH�����ʵ��������
D. ����������μӵķ�Ӧ���������巴Ӧ��Ũ�ȣ�����Ӱٷ�������Ӧ���ʼӿ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�ٴ���ڻ�����ˮ����С�մ�
��1���������������ڻ�������________�����ڼ����________������Ư���Ե���________�����������ᷴӦ���������������Ʒ�Ӧ����________��������ţ�
��2��������ˮ�еĵ��뷽��ʽΪ________��
��3��С�մ���ϡ���ᷴӦ�����ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ��У������ѧ���������ۻ�ѧ�Ծ��������棩 ���ͣ������
2014��7�º�12��ɽ����ѧ���ӿ�ѧ�о����Ի�����ڡ���˼��������廪��ѧ�������ڡ��������ʴ�ѧLai-Sheng Wang���ڼ�������ѧ�����ʽ��ڿ�����������״κϳɡ��й���������ӡ���������ϩB40��B40�Ǽ�C60֮��ڶ�����ʵ�����������ȫȷ�ϵ����ǽ�����״�Ŵء�
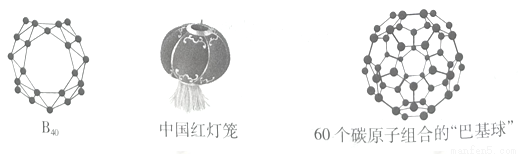
(1) ��̬��ԭ�ӵ���Χ�����Ų�ʽΪ___________��̼60��̼ԭ���ӻ���ʽΪ____________��
(2)����̼60�������������___________________��
������ͬ���ڵ���һ�����ܱ�����Ԫ����___________�֡�
(4)����(BP)������Ԫ������Ԫ����ɵ������������һ�ְ뵼����ϡ�����������廯������廯���������и��·�Ӧ�ϳɡ�BP������B�������������ѻ���Pԭ�������������϶�С�
��д�����廯������廯�Ŀռ乹�ͣ�
���廯��__________�����廯��____________��
�����������������������__________��
�ۼ�����������ԭ�Ӻ���ԭ��֮����������(��������Ϊ478pm)____________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com