
���� ��1�������к��ȵĸ��ϡ��ǿ���ǿ�Ӧ����1molˮ���ų�����������к����Լ��к��ȵ��Ȼ�ѧ����ʽ��
��2����������̼��Ƶ��������㶡������ʵ��������ȼ���ȵĸ�����д��ʾ����ȼ���Ȼ�ѧ����ʽ��
��3�������������ʵ����жϷ�Ӧ���������д�Ȼ�ѧ����ʽ���к���ָ����1molˮ���ų���������
��4������CO��ȼ���ȼ��㣬2molCH4��ȫȼ������Һ̬ˮ�����ų����������ٸ����Ȼ�ѧ����ʽ����дԭ�������д��
��5��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�������������1mol C6H6��l����ȫȼ�շų�������������Ȼ�ѧ����ʽ����д����д���Ȼ�ѧ����ʽ��
��� �⣺��1��1mol H2SO4��Һ������ NaOH��Һ��ȫ��Ӧ���ų�114.6kJ��������������2molˮ�ų�114.6kJ����������Ӧ�ķ�Ӧ��Ϊ-114.6kJ/mol��
�к���Ϊ-57.3kJ/mol�����к��ȵ��Ȼ�ѧ����ʽ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2��n��CaCO3��=$\frac{25g}{100g/mol}$=0.25mol��
Ca��OH��2+CO2=CaCO3+H2O
1 1
0.25mol 0.25mol
������ȼ�����ɵĶ�����̼Ϊ0.25mol��
2C4H10+13O2=8CO2+10H2O
2 8
x 0.25mol
x=$\frac{1}{16}$mol����Ϊȼ��$\frac{1}{16}$mol�Ķ���ų�������ΪQ�������ȼ����Ϊ16Q��
�ʱ�ʾ����ȼ���Ȼ�ѧ����ʽΪ C4H10��g��+$\frac{13}{2}$O2��g��=4CO2��g��+5H2O��l����H=-16QkJ/mol��
�ʴ�Ϊ��C4H10��g��+$\frac{13}{2}$O2��g��=4CO2��g��+5H2O��l����H=-16QkJ/mol��
��3��n��KOH��=$\frac{11.2g}{56g/mol}$=0.2mol��n��H2SO4��=1L��0.1mol/L=0.1mol������ǡ�÷�Ӧ����0.2molˮ���ų�11.46KJ��������
������1molˮʱ�ų�������Ϊ$\frac{1}{0.2}$��11.46kJ=57.3kJ��
�����Ȼ�ѧ����ʽΪ KOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$K2SO4��aq��+H2O��H=-57.3kJ/mol���к���ָ����1molˮ���ų������������к���Ϊ57.3kJ��
�ʴ�Ϊ��KOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$K2SO4��aq��+H2O��H=-57.3kJ/mol��57.3kJ��
��4��CO��ȼ����Ϊ283kJ/mol����ͬ�����£�2molCH4��ȫȼ������Һ̬ˮ�����ų�������Ϊ283kJ��6.3=1782.9kJ���ʼ�����ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪ��CH4��g��+2O2 ��g��=CO2��g��+2H2O��1����H=-891.45kJ/mol��
�ʴ�Ϊ��CH4��g��+2O2 ��g��=CO2��g��+2H2O��1����H=-891.45kJ/mol��
��5��1.00g C6H6��l����O2����ȫȼ������CO2��g����H2O��l�����ų�41.8kJ����������1molC6H6��l������������ȫȼ�շų�������Ϊ41.8��78=3260.4KJ�����C6H6��l����ȼ����Ϊ��H=-3260.4kJ/mol��C6H6��l��ȼ���ȵ��Ȼ�ѧ����ʽΪC6H6��l��+$\frac{15}{2}$O2��g���T6CO2��g��+3H2O��l����H=-3260.4 kJ/mol��
�ʴ�Ϊ��3260.4��C6H6��l��+$\frac{15}{2}$O2��g���T6CO2��g��+3H2O��l����H=-3260.4 kJ/mol��
���� ���⿼���Ȼ�ѧ����ʽ����д�����ø�˹���ɵļ��㣬��Ŀ�Ѷ��еȣ�ע������к��Ⱥ�ȼ���ȵĸ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
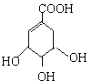 ����ʾ��������
����ʾ�������� 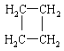 �ɼ�д�ɡ���
�ɼ�д�ɡ���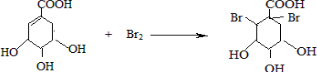 ��
��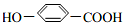 �����䷴Ӧ��������ȥ��Ӧ��
�����䷴Ӧ��������ȥ��Ӧ��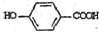 +2Br2��
+2Br2��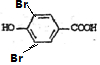 +2HBr��
+2HBr���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KCl��Br2 | B�� | NaCl��KCl | C�� | KI��NaCl | D�� | NaCl��KCl��I2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 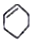 ���ڷ����廯���� ���ڷ����廯���� | B�� | 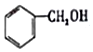 ���ڷ������ ���ڷ������ | ||
| C�� | 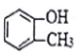 ����֬�������� ����֬�������� | D�� | CH3CH2CH��CH3��2������״������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
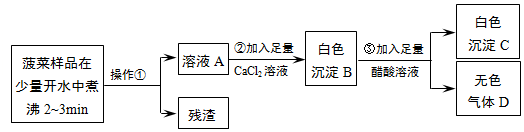
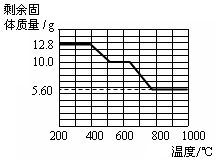
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K-��Ba2+��NO3-��SO32- | B�� | NH4+��Al3+��Cl-��SO42- | ||
| C�� | K Na2+[Al��OH��4]-��SO42- | D�� | Na+��K+��SO42-��Br- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��Ũ�����Cu��ȡSO2 | B�� | ��Ũ�����MnO2��ȡCl2 | ||
| C�� | �ù�������Ͷ���������ȡO2 | D�� | ��NH4C1��Һ��Ca��OH��2��ȡNH3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com