
��12�֣���֪��A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��E��һ�־�����ζ��Һ�������������������л���֮���ת����ϵ��
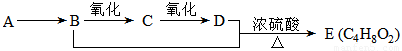
��1��A��E�Ľṹ��ʽ�ֱ�Ϊ __________________ �� ____________________��
��2��A��B�ķ�Ӧ����Ϊ ________________________��
��3��C�����Ĺ���������_______________ �����������õ��Լ�Ϊ ____________��
��4��д��B��C�Ļ�ѧ��Ӧ����ʽ��________________________��
��5����ʵ��������Ҳ������ͼ��ʾ��װ����ȡ�������������б���̼������Һ����Ҫ������_______________________________ ��
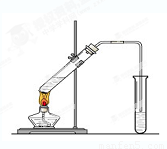
��6��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�_____________________________ ��
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����__
_____��
��1��CH2=CH2 CH3COOCH2CH3 ��2���ӳɷ�Ӧ
��3��ȩ����������Һ������������ͭ����Һ
��4��2CH3CH2OH+O2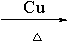 2CH3CHO+2H2O
2CH3CHO+2H2O
��5���кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��6�������� ��7����ֹ���Թ���Һ�屩�С�
�����������������֪A����ϩ����ϩ����̼̼˫��������B�����ʿ�֪����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�������B���Ҵ�����C����ȩ����ȩ�����������ᣬ������Ҵ�����������Ӧ��������������
��3����ȩ�к���ȩ����ȩ���ܷ���������Ӧ������Ƶ�������ͭ����Һ��Ӧ���ݴ˿��Լ���
��5�����ɵ����������к���������Ҵ������ñ���̼������Һ��ȥ�����������кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��6���Ҵ��������ˮ���ǻ��ܵģ����Ե��ܿ�ֱ�Ӳ���ˮ�У���������Һ�嵹����
��7�����ڷ�Ӧ��Ҫ���ȣ����Լ������Ƭ�ܷ�ֹ���Թ���Һ�屩�С�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Cu |
| �� |
| Cu |
| �� |
| ���� |
| �ƻ�ø |
| ���� |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
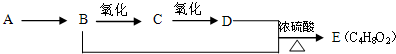
��12�֣���֪��A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��E��һ�־�����ζ��Һ�������������������л���֮���ת����ϵ��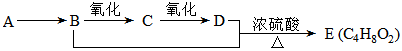
��1��A��E�Ľṹ��ʽ�ֱ�Ϊ__________________��____________________��
��2��A��B�ķ�Ӧ����Ϊ________________________��
��3��C�����Ĺ���������_______________�����������õ��Լ�Ϊ____________��
��4��д��B��C�Ļ�ѧ��Ӧ����ʽ��________________________��
��5����ʵ��������Ҳ������ͼ��ʾ��װ����ȡ�������������б���̼������Һ����Ҫ������_______________________________ ��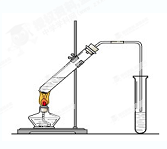
��6��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�_____________________________ ��
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����__
_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008-2009ѧ��ɽ��ʡ�ൺ�и߶����£���ĩ��ѧ�Ծ��������棩 ���ͣ������
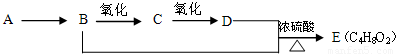
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��E��һ�־�����ζ��Һ�������������������л���֮���ת����ϵ��
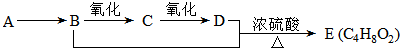
��1��A��E�Ľṹ��ʽ�ֱ�Ϊ �� ��
��2��A��B�ķ�Ӧ����Ϊ ��
��3��C�����Ĺ��������� �����������õ��Լ�Ϊ ��
��4��д��B��C�Ļ�ѧ��Ӧ����ʽ�� ��
��5����ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�������������б���̼������Һ����Ҫ������_____________ ____________________ ��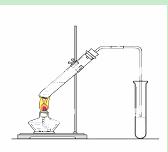
��6��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ_____________________________ ��
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����__ _____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com