
·ÖĪö £Ø1£©£Ø2£©ŅĄ¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčŃ”ŌńŠčŅŖŅĒĘ÷£»
£Ø3£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬¾Ż“ĖÅÅŠņ£»
£Ø4£©·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£»
£Ø5£©ŅĄ¾Żm=CVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ森
½ā“š ½ā£ŗ£Ø1£©£Ø2£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬ĖłŅŌŠčŅŖŅĒĘ÷£ŗĶŠÅĢĢģĘ½”¢Ōæ³×”¢ÉÕ±”¢ĮæĶ²”¢²£Į§°ō”¢ČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬ÅäÖĘ100mL 2mol•L-1µÄNaOHČÜŅŗ£¬Ó¦Ń”Ōń100mLČŻĮæĘ棬ĖłŅŌÓĆ²»µ½µÄŅĒĘ÷£ŗA£»
»¹Č±ÉŁµÄŅĒĘ÷£ŗÉÕ±”¢²£Į§°ō£»
¹Ź“š°øĪŖ£ŗ£Ø1£©A£»£Ø2£©ÉÕ±”¢²£Į§°ō£»
£Ø3£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬ĖłŅŌÕżČ·µÄĖ³ŠņĪŖ£ŗBEAGCDF£»
¹Ź“š°øĪŖ£ŗBEAGCDF£»
£Ø4£©A£®¶ØČŻŹ±ŃöŹÓČŻĮæĘææĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹA²»Ń”£»
B£®ČŻĮæĘæÖŠÓŠÉŁĮæĖ®£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»»į²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹ŹB²»Ń”£»
C£®Ī“ĄäČ“¶ØČŻ£¬ĄäČ“ŗóČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹCŃ”£»
D£®¶ØČŻŗ󣬰ŃČŻĮæĘæµ¹ÖĆŅ”ŌČŗó·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬±ć²¹³ä¼øµĪĖ®ÖĮæĢ¶Č“¦£¬µ¼ÖĀµ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹD²»Ń”£»
¹ŹŃ”£ŗC£®
£Ø5£©ÓĆNaOH¹ĢĢåÅäÖĘ100mL 2mol•L-1µÄNaOHČÜŅŗ£¬ŠčŅŖĒāŃõ»ÆÄĘÖŹĮæm=0.1L”Į2mol/L”Į40g/mol=8.0g£»
¹Ź“š°øĪŖ£ŗ8.0£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬Ć÷Č·ÅäÖĘŌĄķ¼°²Ł×÷²½ÖčŹĒ½āĢā¹Ų¼ü£¬×¢ŅāŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪöµÄ·½·ØŗĶ¼¼ĒÉ£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
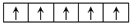 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
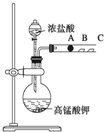 Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼ĘĮĖŅ»×鏵Ń饓Ģ½¾æŌŖĖŲÖÜĘŚĀÉ£®¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼×°ÖĆĄ“ŃéÖ¤Ā±×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ£®A”¢B”¢CČż“¦·Ö±šŹĒÕ“ÓŠNaBrČÜŅŗµÄĆŽ»Ø”¢ŹŖČóµÄµķ·ŪKIŹŌÖ½”¢ŹŖČóŗģÖ½£®ŅŃÖŖ³£ĪĀĻĀÅØŃĪĖįÓėøßĆĢĖį¼ŲÄÜ·“Ӧɜ³ÉĀČĘų£®
Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼ĘĮĖŅ»×鏵Ń饓Ģ½¾æŌŖĖŲÖÜĘŚĀÉ£®¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼×°ÖĆĄ“ŃéÖ¤Ā±×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ£®A”¢B”¢CČż“¦·Ö±šŹĒÕ“ÓŠNaBrČÜŅŗµÄĆŽ»Ø”¢ŹŖČóµÄµķ·ŪKIŹŌÖ½”¢ŹŖČóŗģÖ½£®ŅŃÖŖ³£ĪĀĻĀÅØŃĪĖįÓėøßĆĢĖį¼ŲÄÜ·“Ӧɜ³ÉĀČĘų£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 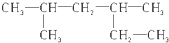 2£¬4-¶ž¼×»ł¼ŗĶé 2£¬4-¶ž¼×»ł¼ŗĶé | |
| B£® | 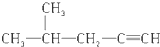 2-¼×»ł-4-ĪģČ² 2-¼×»ł-4-ĪģČ² | |
| C£® | 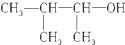 3-¼×»ł-2-¶”“¼ 3-¼×»ł-2-¶”“¼ | |
| D£® | CH3-CHBr-CHBr-CH3 2£¬3-¶žä嶔Ķé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÉśŹÆ»Ņ | B£® | ľĢæ | C£® | ĮņĖį±µ | D£® | ĀČ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ū | B£® | ¢Ł¢Ś¢Ū | C£® | ¢Ū¢Ü | D£® | ¢Ś¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com